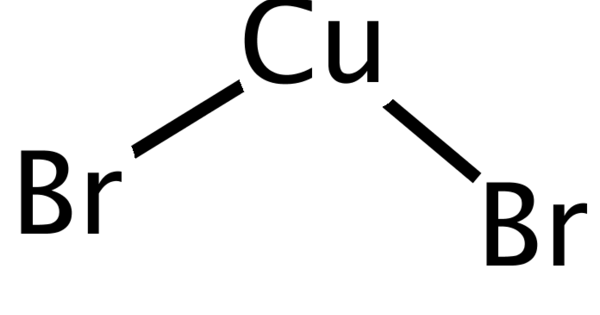
Copper(II) bromide (CuBr2) is a chemical compound. It is an odorless black solid. It is used as an intensifier in photography and as a brominating agent in organic synthesis. It is also used in copper vapor lasers, a type of laser in which the medium is copper bromide vapour formed in-situ from hydrogen bromide reacting with the copper discharge tube. It is used in dermatological applications to produce yellow or green light.
Properties
- Chemical formula: CuBr2
- Molar mass: 223.37 g/mol
- Appearance: grayish black crystals deliquescent
- Density: 4.710 g/cm3, solid
- Melting point: 498 °C (928 °F; 771 K)
- Boiling point: 900 °C (1,650 °F; 1,170 K)
- Solubility in water: 55.7 g/100 mL (20 °C)
- Crystal structure: monoclinic

Synthesis
Copper(II) bromide can be obtained by combining copper oxide and hydrobromic acid:
CuO + 2HBr → CuBr2 + H2O.
Purification
Copper(II) bromide is purified twice from water, then filtered to remove any CuBr and concentrated under vacuum. Using phosphorus pentoxide, this product is dehydrated.
Reactions
Copper (II) bromide reacts with ketones in chloroform-ethyl acetate to produce alpha-bromo ketones. The finished product can be used directly to make derivatives. According to reports, this heterogeneous method is the most selective and direct method of forming α-bromo ketones.
In order for an NPG to serve as a glycosyl acceptor during halonium-promoted couplings, dibromination of NPGs, n-pentenyl glycosides, using a CuBr2/LiBr reagent combination was performed. A high yield of dibromides from alkenyl sugars that are resistant to direct reaction with molecular bromine is obtained in this manner.
Usage
Copper(II) bromide lasers, which emit pulsed yellow and green light, have been investigated as a potential treatment for cutaneous lesions. Copper bromide treatment has also been shown in studies to be beneficial for skin rejuvenation. Its solution was widely used in photography as a bleaching step for intensifying collodion and gelatin negatives. Copper(II)bromide has also been proposed for use in humidity indicator cards.
Safety
If copper(II) bromide is swallowed, it is not harmful. It has no effect on the central nervous system, the brain, the eyes, the liver, or the kidneys. It does not irritate the skin, eyes, or respiratory tract. In fact, if consumed in large quantities, it has been shown to have therapeutic effects.