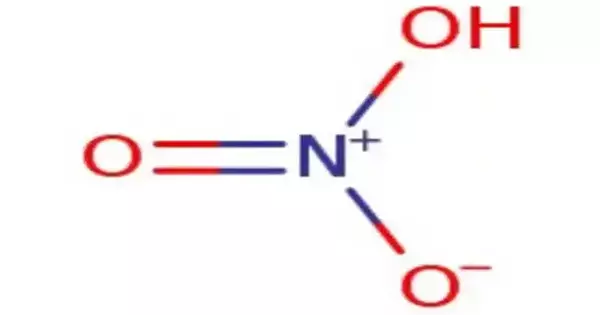
Nitric acid is an inorganic chemical having the formula HNO3. It is a highly corrosive and potent mineral acid. It is an extremely corrosive mineral acid. It is a colorless liquid at room temperature and is extensively employed in numerous industrial processes, laboratory investigations, and as a component in the creation of fertilizers and explosives. The chemical is colorless, although samples tend to acquire a yellow tinge over time due to breakdown into nitrogen oxides.
The majority of commercially available nitric acid has a water content of 68%. Fumigating nitric acid is a solution that contains more than 86% HNO3. Fumigating nitric acid is classified as red-fuming nitric acid at concentrations over 86% or white-fuming nitric acid at concentrations above 95%, depending on the amount of nitrogen dioxide present.
Properties
- Chemical Formula: HNO3
- Molecular Weight: Approximately 63.01 g/mol
- Appearance: Colorless liquid
- Odor: Pungent and suffocating
- Density: Approximately 1.51 g/cm³
- Boiling Point: 83°C (181.4°F)
- Melting Point: -42°C (-43.6°F)
Nitric acid is the most common reagent used in nitration, which involves the addition of a nitro group to an organic molecule. While some of the resulting nitro compounds are shock- and thermally-sensitive explosives, others are stable enough to be employed in munitions and demolition, while still, others are stable enough to be utilized as pigments in inks and dyes. Nitric acid is also often employed as a powerful oxidizing agent.
Chemical Properties:
Nitric acid is a powerful acid that dissociates easily in water, releasing hydrogen ions (H+). It is a strong oxidizing agent that can react violently with reducing chemicals. It can generate nitrates when it reacts with various metals.
Uses
- Chemical Synthesis: It is used in the synthesis of various chemicals, including explosives like nitroglycerin and trinitrotoluene (TNT).
- Fertilizer Production: It is a key component in the manufacture of ammonium nitrate, a common nitrogen-based fertilizer.
- Laboratory Reagent: It is used in laboratories for various analytical and chemical testing procedures.
- Etching: It is employed in metal etching processes, particularly for the preparation of electronic components.
- Pickling: In industries such as steel manufacturing, nitric acid is used for pickling stainless steel and other metals to remove scale and impurities.
- Rocket Propulsion: In the aerospace industry, concentrated nitric acid is used as a rocket propellant oxidizer.
- Cleaning Agent: Diluted nitric acid solutions are used for cleaning and passivating stainless steel and other materials.
Safety Precautions:
Nitric acid is extremely corrosive and must be handled with utmost caution. Because it can inflict serious burns to the skin and eyes, gloves and goggles should be worn. To avoid unintended reactions, it should be stored away from incompatible compounds, particularly reducing agents and organic molecules.
Overall, because of its strong acidic and oxidizing capabilities, nitric acid is essential in many industrial processes and chemical applications, but it should be used and handled with extreme caution due to its corrosive nature.