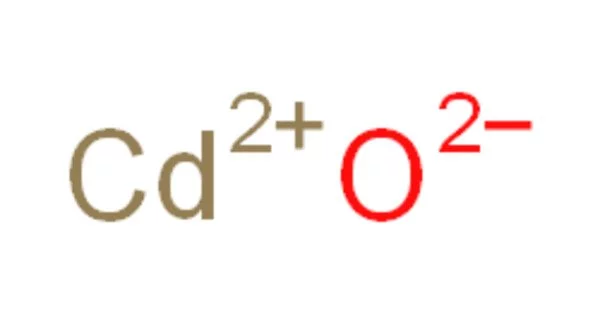Cadmium oxide, abbreviated CdO, is an inorganic compound. It is a dark brown crystalline inorganic compound that emits toxic cadmium oxide fumes when heated. It is a major precursor to other cadmium compounds. Like sodium chloride, it crystallizes in a cubic rocksalt lattice with octahedral cation and anion centers. It can be found in nature as the rare mineral monteponite. Cadmium oxide is available as a colorless amorphous powder as well as brown or red crystals.
Cadmium oxide is an n-type semiconductor with a room-temperature band gap of 2.18 eV. It is primarily used in nickel-cadmium batteries, but it is also used in electroplating, plastics, and nitrile rubber production, and as a nematocide and ascaricide in swine.
Properties
Cadmium oxide appears as brown crystals or brown amorphous powder. Used as an electroplating chemical and in the manufacture of cadmium electrodes. Is a component of silver alloys, phosphors, semiconductors, glass, and ceramic glazes.
- Chemical formula: CdO
- Molar mass: 128.413 g·mol−1
- Appearance: colorless powder (alpha form); red-brown crystal (beta form)
- Odor: odorless
- Density: 8.15 g/cm3(crystalline), 6.95 g/cm3 (amorphous) solid.
- Melting point: 900–1,000 °C; decomposition of amorphous form
- Boiling point: 1,559 °C (2,838 °F; 1,832 K) sublimation
- Solubility in water: 4.8 mg/L (18 °C)
- Solubility: soluble in dilute acid slowly soluble in ammonium salts
- Vapor pressure: 0.13 kPa (1000 °C)
- Band gap: 2.18 eV

Production and structure
Cadmium oxide is a common byproduct of zinc refining because cadmium compounds are frequently found in association with zinc ores. It is made by igniting elemental cadmium in air. This oxide can also be obtained by pyrolysis of other cadmium compounds, such as nitrate or carbonate. When pure, it is red, but CdO is unusual in that it is available in a wide range of colors due to its proclivity to form defect structures caused by anion vacancies. Cadmium oxide is commercially produced by oxidizing cadmium vapor in air.
Uses
Cadmium oxide is used in cadmium plating baths, storage battery electrodes, cadmium salts, catalyst, ceramic glazes, phosphors, and nematocide. Cadmium oxide is commonly used in electroplating baths, optoelectronic devices, and pigments.
- Transparent conductor
CdO is a transparent conductive material that was first prepared as a transparent conducting film by Karl Baedeker in 1907. Cadmium oxide thin films have been used in photodiodes, phototransistors, photovoltaic cells, transparent electrodes, liquid crystal displays, infrared detectors, and anti-reflection coatings.
- Cadmium plating
Most commercial electroplating of cadmium is done by electrodeposition from cyanide baths. These cyanide baths consist of cadmium oxide and sodium cyanide in water, which likely form cadmium cyanide and sodium hydroxide. A typical formula is 32 g/L cadmium oxide and 75 g/L sodium cyanide.
Health Hazards
Cadmium compounds are known carcinogens. This substance irritates the eyes, skin, and respiratory tract, damages the lungs, causing shortness of breath, chest pain, and pulmonary edema, and can also damage the kidneys, causing proteinuria and decreased renal function. It is a known carcinogen and has been linked to an increased risk of developing lung cancer.