Pentaborane (9), with the formula B5H9, is an inorganic compound. Despite being a highly reactive compound, it is one of the most common boron hydride clusters. It was once considered as rocket or jet fuel due to its high reactivity to oxygen. Pentaborane, like many of the smaller boron hydrides, is colorless, diamagnetic, and volatile. It has a connection to pentaborane(11) (B5H11).
Pentaboron nonahydride, stable pentaborane, or pentaborane(9) is a chemical compound that was considered a good prospect for a rocket or jet fuel by both the US and Russian armed services in the 1950s, a so-called “exotic fuel.” It is one of the boranes, with a chemical structure of five atoms of boron and nine atoms of hydrogen (B5H9). Because simple boron compounds burn with a characteristic green flame, the nickname for this fuel in the U.S. industry was “Green Dragon”.
Properties
Pentaborane is a colorless mobile liquid with a strong pungent odor. It is a clear colorless liquid with a pungent odor like sour milk. Its freezing point -52.9°F (-46.6°C). Its boiling point 136.4°F (58°C). It’s decomposition temperature 302°F (150°C). Above 30 °C it can form explosive concentration of vapors with air. Its vapors are heavier than air. It is pyrophoric – can ignite spontaneously in contact with air, when even slightly impure.
- Chemical formula: (B5H9)
- Molar mass: 63.12 g/mol
- Appearance: Colorless liquid
- Odor: pungent, like sour milk
- Density: 0.618 g/mL
- Melting point: −46.8 °C (−52.2 °F; 226.3 K)
- Boiling point: 60.1 °C (140.2 °F; 333.2 K)
- Solubility in water: Reacts with water
- Vapor pressure: 171 mmHg (20°C)
Structure and synthesis
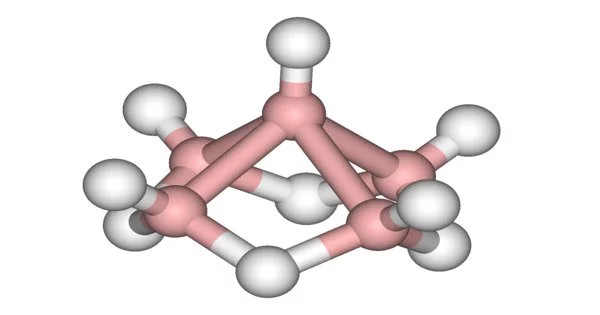
Its structure is made up of five boron atoms arranged in a square pyramid. Each boron has a terminal hydride ligand, and four hydrides span the pyramid’s base. It’s known as a nido cage.
Alfred Stock invented it by pyrolyzing diborane at around 200 °C. An improved synthesis begins with octahydrotriborate (B3H8) salts, which are converted to the bromide B3H7Br using HBr. Pentaborane is produced by the pyrolysis of this bromide.
5 B3H7Br– → 3 B5H9 + 5 Br– + 4 H2
Callery Chemical Company manufactured pentaborane on a commercial scale in the United States.
It decomposes at temperatures above 150 °C, producing hydrogen; when this occurs in a closed container, the resulting pressure rise can be dangerous. It is significantly more stable in the presence of water than diborane. It is soluble in hydrocarbons, benzene, and cyclohexane, as well as greases used in laboratory equipment. It decomposes slowly in storage, producing only a trace of hydrogen and solid residue.
Reactions
The chemistry of pentaborane is complex. Halogenation produces the symmetrical derivatives B5H8X, which can be isomerised to position the halide at the base of the square pyramid. It can be deprotonated with strong bases such as alkyl lithium reagents, and the resulting lithium salts react with various electrophiles to give substituted derivatives. It is Lewis acidic, forming double adducts with two trimethylphosphine equivalents. Pentaborane is used to create other boron hydride clusters.
Safety
As one of the compounds that have a NFPA 704 (fire diamond) rating of 4 for every category, it is naturally extremely dangerous.
It can form an explosive concentration of vapors with air at temperatures above 30 °C. Its vapors weigh more than air. It is pyrophoric, meaning it can spontaneously ignite when it comes into contact with air, even if it is slightly impure. It can also easily form shock sensitive explosive compounds and reacts violently with some fire extinguishants, particularly halocarbons and water. It is extremely toxic, and symptoms of lower-level exposure can appear up to 48 hours later. It has acute toxicity similar to some nerve agents.
The Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have set occupational exposure limits for pentaborane at 0.005 ppm (0.01 mg/m3) over an eight-hour time-weighted average, with a short-term exposure limit of 0.015 ppm (0.03 mg/m3). The acute toxicity of pentaborane has caused it to be considered immediately dangerous to life and health, with a limit set at 1 ppm.