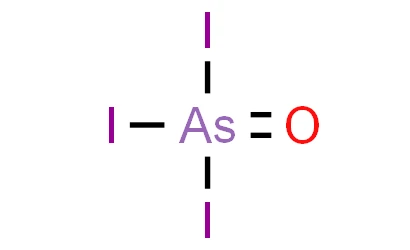Arsenic triiodide is an inorganic compound with the formula AsI3. It is a compound composed of arsenic and iodine. It is an orange to dark red solid that readily sublimes. It is a pyramidal molecule that is useful for preparing organoarsenic compounds. It is a rare and highly reactive solid, typically appearing as bright red or reddish-brown crystals.
Arsenic triiodide is highly reactive and unstable, particularly in the presence of moisture or oxygen. It decomposes readily, releasing toxic arsenic and iodine vapors. Due to its instability and toxicity, arsenic triiodide has limited practical applications. However, it has been used in chemical research as a reagent in certain organic synthesis reactions.
Properties
Arsenic triiodide typically exists as bright red or reddish-brown crystals. These crystals may darken upon exposure to light and air due to decomposition. It is sparingly soluble in water, meaning it dissolves only to a small extent. It is more soluble in organic solvents like carbon disulfide and chloroform.
- Chemical formula: AsI3
- Molar mass: 455.635 g/mol
- Appearance: orange-red crystalline solid
- Density: 4.69 g/cm3
- Melting point: 146 °C (295 °F; 419 K)
- Boiling point: 403 °C (757 °F; 676 K)
- Solubility in water: 6 g/100 mL
- Solubility: soluble in alcohol, ether, CS2; dissolves in chloroform, benzene, toluene
- Magnetic susceptibility (χ): -142.0·10−6 cm3/mol
Preparation
Arsenic triiodide can be prepared by the reaction of arsenic with iodine in the presence of a suitable solvent. The reaction typically involves heating and careful handling due to the compound’s reactivity. It is prepared by a reaction of arsenic trichloride and potassium iodide:
AsCl3 + 3KI → AsI3 + 3 KCl
Reactions
Hydrolysis occurs only slowly in water forming arsenic trioxide and hydroiodic acid. The reaction proceeds via the formation of arsenic acid which exists in equilibrium with hydroiodic acid. The aqueous solution is highly acidic, pH of 0.1N solution is 1.1. It decomposes to arsenic trioxide, elemental arsenic, and iodine when heated in air at 200 °C. The decomposition, however, commences at 100 °C and occurs with the liberation of iodine.
Toxicity
Like other arsenic compounds, arsenic triiodide is toxic. Exposure to arsenic compounds can lead to various health issues, including skin lesions, respiratory problems, and even death in extreme cases.
Storage
Arsenic triiodide should be stored in airtight containers away from moisture and light to minimize decomposition. Proper handling procedures should be followed due to its toxicity and reactivity. Due to its hazardous nature and limited practical uses, arsenic triiodide is not commonly encountered outside of specialized laboratory settings where strict safety protocols are followed.