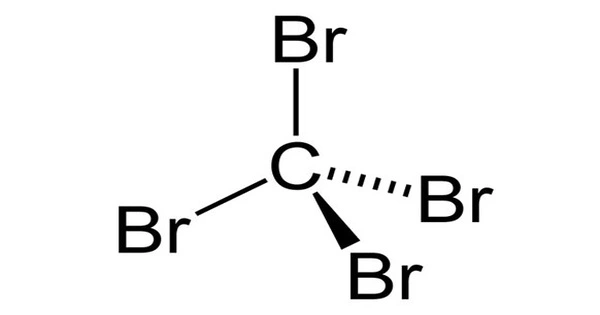
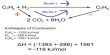
Carbon tetrabromide, CBr4, is a carbon bromide that is also known as tetrabromomethane. It has the appearance of a colorless crystalline solid. It has a much higher density than water and is insoluble in water. It is a highly toxic chemical that can be fatal if inhaled, swallowed, or absorbed through the skin. It will not adsorb to suspended solids or sediment in the water column. It has a moderate potential for bioconcentration in aquatic organisms.
Physical properties
At room temperature, carbon tetrabromide is a colorless, nonflammable solid. It is insoluble in water but soluble in a variety of organic solvents including alcohol, ether, and chloroform. The specific gravity is 3.42, the melting point is 90°C, the boiling point is 189°C, and the vapor pressure at 25°C is 0.72 torr. It has a high mobility in soil and volatilizes slowly from the dry soil surface. Its biodegradation is expected to be slow, and it will only exist as a vapor in the surrounding atmosphere.
- Chemical formula: CBr4
- Molar mass: 331.627 g·mol−1
- Appearance: Colorless to yellow-brown crystals
- Odor: sweet odor
- Density: 3.42 g mL−1
- Melting point: 94.5 °C; 202.0 °F; 367.6 K
- Boiling point: 189.7 °C; 373.4 °F; 462.8 K decomposes
- Solubility in water: 0.024 g/100 mL (30 °C)
- Solubility: soluble in ether, chloroform, ethanol
- Vapor pressure: 5.33 kPa (at 96.3 °C)
- Crystal structure: Monoclinic

Plastic crystallinity
A plastic crystal phase is the high-temperature α phase. In an fcc arrangement, the CBr4 are roughly located on the corners of the cubic unit cell as well as on the centers of its faces. Previously, it was thought that the molecules could rotate more or less freely (a ‘rotor phase’), giving them the appearance of spheres on average. However, recent research has revealed that the molecules are limited to only six possible orientations (Frenkel disorder).
Furthermore, they cannot take these orientations completely independently of each other because the bromine atoms of neighboring molecules would point at each other in some cases, resulting in impossibly short distances.
When two neighbor molecules are considered, this eliminates certain orientational combinations. Even for the remaining combinations, displacive changes occur that better accommodate neighbor-to-neighbor distances. The combination of censored Frenkel disorder and displacive disorder implies a significant amount of disorder inside the crystal, resulting in highly structured sheets of diffuse scattered intensity in X-ray diffraction. In fact, it is the structure in the diffuse intensity that provides information about the structure’s details.
Chemical reactions
CBr4 is used in the Appel reaction, which converts alcohols to alkyl bromides, in combination with triphenylphosphine. CBr4 is also used in the first step of the Corey-Fuchs reaction, which converts aldehydes to terminal alkynes, in conjunction with triphenylphosphine. It has a much lower stability than lighter tetrahalomethanes. It’s created by brominating methane with HBr or Br2. It can also be made more cheaply by combining tetrachloromethane and aluminum bromide at 100°C.
Uses
It is used as a solvent for greases, waxes, and oils, as well as in the plastic and rubber industries for blowing and vulcanization, polymerization, as a sedative, and as an intermediate in the production of agrochemicals. It is used as an ingredient in fire-resistant chemicals due to its non-flammability. Because of its high density, it is also used for mineral separation.