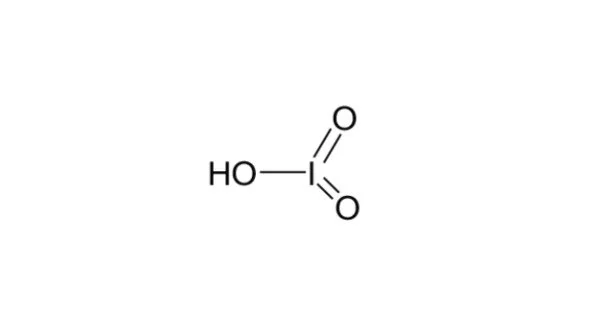
Iodic acid has the chemical formula HIO3 and is a white water-soluble solid. Its stability contrasts with the instability of chloric and bromic acids. It is an iodine oxoacid with a structure similar to other oxoacids such as sulfuric acid and phosphoric acid. Iodic acid is made up of three atoms: hydrogen (H), iodine (I), and oxygen (O).
Iodic acid contains iodine in the oxidation state +5 and is one of the most stable halogen oxo-acids. Iodine pentoxide is produced when samples are heated. When heated further, the iodine pentoxide decomposes further, yielding a mixture of iodine, oxygen, and lower iodine oxides. Because of its chemical properties, it can be used as a reagent in a variety of chemical reactions and analytical chemistry methods.
Properties
With a pKa of 0.75, iodic acid is a relatively strong acid. It oxidizes strongly in acidic solutions but less so in basic solutions. When iodic acid acts as an oxidizer, the reaction produces either iodine or iodide ion. Iodic acid is reduced to iodine trichloride, a golden yellow compound in solution, under certain conditions (very low pH and high concentration of chloride ions, such as in concentrated hydrochloric acid) and no further reduction occurs.
- Chemical formula: HIO3
- Molar mass: 175.91 g/mol
- Appearance: White solid
- Density: 4.62 g/cm3, solid
- Melting point: 110 °C (230 °F; 383 K)
- Solubility in water: 269 g/100 mL (20 °C)
- Acidity (pKa): 0.75
- Conjugate base: Iodate
- Magnetic susceptibility (χ): −48.0·10−6 cm3/mol
Preparation
Iodic acid can be produced by oxidizing iodine I2 with strong oxidizers such as nitric acid HNO3, chlorine Cl2, chloric acid HClO3 or hydrogen peroxide H2O2, for example:
I2 + 6H2O + 5Cl2 ↔ 2 HIO3 + 10 HCl
Structure
Iodic acid crystallises from acidic solution as orthorhombic α-HIO
3 in space group P212121. The structure consists of pyramidal molecules linked by hydrogen bonding and intermolecular iodine-oxygen interactions. The I=O bond lengths are 1.81 Å while the I–OH distance is 1.89 Å. Several other polymorphs have been reported, including an orthorhombic γ form in space group Pbca and an orthorhombic δ form in space group P212121. All of the polymorphs contain pyramidal molecules, hydrogen bonding and I···O interactions, but differ in packing arrangement.
Uses
In analytical chemistry, iodic acid is used as a strong acid. It can be used to standardize both weak and strong base solutions by using methyl red or methyl orange as the indicator.
To increase the iodine content of salt, iodic acid can be used to synthesize sodium or potassium iodate. It can be used in chemical reactions as an oxidizing agent. It has the ability to convert certain compounds into higher oxidation states. It is frequently used to make iodine compounds such as potassium iodate (KIO3), which is used to make iodized salt.