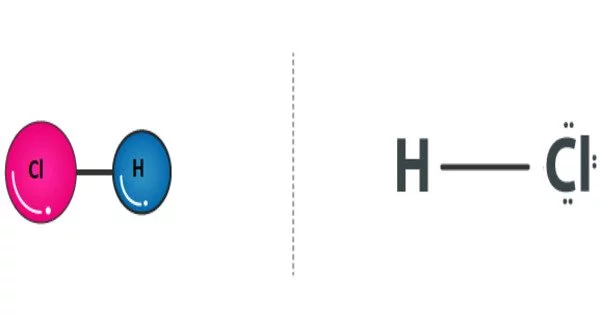
The compound hydrogen chloride has the chemical formula HCl and is thus a hydrogen halide. It is a colorless gas at room temperature that emits white hydrochloric acid fumes when it comes into contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. The aqueous solution of hydrogen chloride, hydrochloric acid, is also known as HCl.
Properties
HCl is highly soluble in water, and when dissolved, it forms hydrochloric acid. It is highly corrosive to metals, and it can cause severe burns on contact with the skin or eyes.
- Chemical formula: HCl
- Molar mass: 36.46 g/mol
- Appearance: Colorless gas
- Odor: pungent; sharp and burning
- Density: 1.49 g/L
- Melting point: −114.22 °C (−173.60 °F; 158.93 K)
- Boiling point: −85.05 °C (−121.09 °F; 188.10 K)
- Solubility in water: 823 g/L (0 °C); 561 g/L (60 °C)
- Solubility: soluble in methanol, ethanol, ether
- Vapor pressure: 4352 kPa (at 21.1 °C)
Production
On an industrial scale, HCl is produced using a variety of methods, including the direct reaction of hydrogen and chlorine gases or the reaction of certain chlorine-containing compounds with hydrogen.
Dissolving gaseous hydrogen chloride in water yields hydrochloric acid. Because of the acid’s corrosive nature, ceramic, glass, or occasionally tantalum apparatus is commonly used. Hydrochloric acid is typically sold as a solution containing 28-35 percent hydrogen chloride by weight, also known as concentrated hydrochloric acid. Although anhydrous liquid hydrogen chloride is available, its use is limited due to the large and expensive containers required to store it.
Uses: Hydrogen chloride has numerous applications, including:
- It is widely used in the production of hydrochloric acid, which finds application in various industries, including chemical synthesis, metal cleaning, and ore processing.
- It is used to adjust the pH of solutions in laboratory settings and industrial processes.
- HCl is employed in the production of various chemicals, such as vinyl chloride (used for manufacturing PVC), chlorinated solvents, and dyes.
- It is used for cleaning and pickling metal surfaces to remove rust, scale, and other impurities.
- It may be used for food processing purposes, such as pH adjustment or as an acidifier.