A group of scientists from China, the Netherlands, and Saudi Arabia used a novel type of electron microscopy to measure weak van der Waals interactions. The group describes developing what they call a “molecular compass” to measure weak van der Waals interactions using a new type of electron microscopy developed in the Netherlands in their paper published in the journal Nature.
Van der Waals forces are electrostatic forces that exist between uncharged molecules due to the interaction of electric dipole moments; measuring them typically necessitates the use of highly sophisticated equipment. The researchers have developed a new method for measuring their interactions using less sophisticated equipment in this new effort.
A team of researchers has used a new kind of electron microscopy to measure weak van der Waals interactions.
The work was made possible by the recent development of a new type of electron microscope by a team in the Netherlands. Officially known as integrated differential phase-contrast scanning transmission electron microscopy, the new technology uses image data to create images at the atomic level, yielding results with higher signal-to-noise ratios. This means that smaller electron doses can be used than with other electron microscopes.
The researchers used ZSM-5, a type of zeolite with rings of oxygen atoms and silicon that link around holes in lattice sheets, to measure van der Waals interactions. They stacked several sheets, aligning them in such a way that small channels were formed. The team then used a centrifuge to insert para-xylene molecules into the channels. The para-xylene molecules were then used as a pointer in a type of compass. They discovered that moving the molecules in relation to the oxygen and silicon atoms caused changes in the weak van der Waals interactions. They used the new electron microscope’s imaging capabilities to measure these shifts.
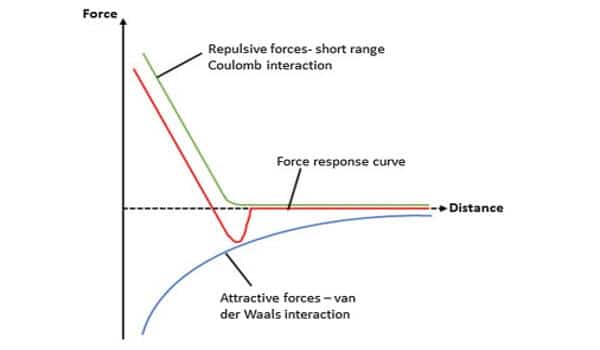
Van der Waals forces are caused by temporary fluctuations in the charge density of neighboring atoms or molecules. Although they are only effective over a short distance, they are common in nature and widely used in industry, where they play critical roles in fields ranging from condensed matter physics to structural biology. Direct measurement, on the other hand, usually necessitates sophisticated techniques best suited to the study of single atoms.
The researchers put their technique to the test by comparing changes in the orientation of the para-xylene pointers to changes in the shape of the rings. They believe their technique could be used to improve applications such as those involved in the conversion of alcohol to gasoline.
Single molecule in a crystal
The Tsinghua team’s experiment uses single molecules of para-xylene (eight carbon and ten oxygen atoms each) trapped within the voids of a zeolite crystal, which is very sensitive to electron beams. (Zeolites are microporous minerals that occur naturally but are also mass-produced industrially.) The zeolite used in this study, ZSM-5, is made up of rings of silicon and oxygen atoms that connect around large holes in a two-dimensional lattice sheet. When several of these sheets are stacked on top of one another, the holes align, resulting in shallow channels running through the structure. It was into these channels that Wei and colleagues placed their para-xylene molecules by mixing ZSM-5 powder and para-xylene liquid in a centrifuge.
Wei and colleagues used the fact that the perimeter of each hole is made up of a ring of 10 silicon and 10 oxygen atoms interspersed at roughly equal intervals of 18 degrees to create their compass. The idea was to use the para-xylene molecule contained within each ring as a pointer. Because any shift in the molecule’s axis relative to the silicon and oxygen atoms (the “compass points”) indicates changes in local van der Waals interactions, the researchers were able to determine the nature of these changes by imaging the molecule and observing the orientation of its long axis in the plane of the ring.
Tiny force changes
Wei and colleagues demonstrated that they could use their compass to measure force changes in both space and time. They accomplished this by comparing changes in the orientation of the para-xylene pointers, as seen in the iDPC-STEM images, to variations in the shape of the (slightly elliptical) rings. These variations were measured using intensity measurements to determine the distance between pairs of atoms on opposite sides of the rings.
The researchers compared multiple rings and discovered that the pointers in each ellipse tend to line up along the major axis. They also discovered that the pointer in any given ring moves between compass points in order to remain aligned with the longest axis as the ring changes shape (as a result of increasing exposure to electrons).
To relate these responses to changes in van der Waals interactions, the researchers used first principles calculations to determine how the interaction energy of para-xylene molecules should vary with ring geometry. They found that for each different ellipse, it was always energetically favourable for the molecule to line up along the longest axis – demonstrating, they say, that each molecule in its crystal void does indeed function as a “van der Waals compass”.