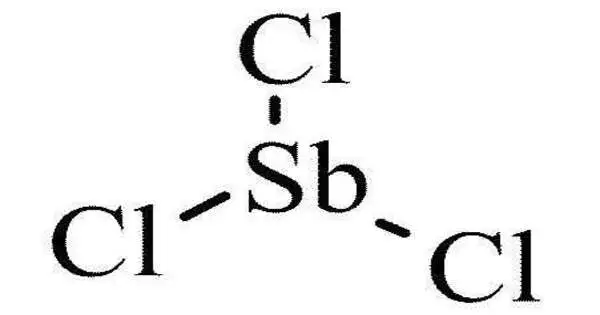Antimony trichloride is a chemical compound with the formula SbCl3. It is a soft colorless solid with a pungent odor and was known to alchemists as butter of antimony. It is a colorless to pale yellow solid that is highly soluble in water. It is commonly used as a reagent in chemical synthesis, particularly in the production of certain dyes and pharmaceuticals.
Antimony trichloride is a colorless to pale yellow liquid at room temperature, although it can also exist as a white solid if it’s kept at very low temperatures. It has a pungent, acrid odor resembling that of hydrochloric acid. It is highly soluble in chlorinated solvents such as carbon tetrachloride and chloroform. It is also soluble in nonpolar solvents like benzene and toluene, but it is only sparingly soluble in water.
Properties
- Chemical formula: Cl3Sb
- Molar mass: 228.11 g·mol−1
- Appearance: Colorless solid, very hygroscopic
- Odor: Sharp, pungent
- Density: 3.14 g/cm3 (25 °C); 2.51 g/cm3 (150 °C)
- Melting point: 73.4 °C (164.1 °F; 346.5 K)[5]
- Boiling point: 223.5 °C (434.3 °F; 496.6 K)
- Solubility in water: 601.1 g/100 ml (0 °C); 985.1 g/100 mL (25 °C)
Preparation
Antimony trichloride is prepared by reaction of chlorine with antimony, antimony tribromide, antimony trioxide, or antimony trisulfide. It also may be made by treating antimony trioxide with concentrated hydrochloric acid.
Uses
Antimony trichloride is used as a catalyst in the production of polyester. It is utilized in the manufacturing of certain dyes, particularly for textiles. It serves as a Lewis acid catalyst in organic synthesis.
In the pharmaceutical industry, it’s used in the production of some medicines. It is also employed in the production of flame retardants. It has also been used as an adulterant to enhance the louche effect in absinthe. It has been used in the past to dissolve and remove horn buds from calves without having to cut them off.
Hazards
Antimony trichloride is toxic if inhaled, ingested, or absorbed through the skin. It can cause irritation to the respiratory system, skin, and eyes upon exposure. Long-term exposure may lead to more serious health effects. It is corrosive to metals and may cause skin irritation and respiratory issues upon exposure. Proper safety measures should be taken when handling this compound.