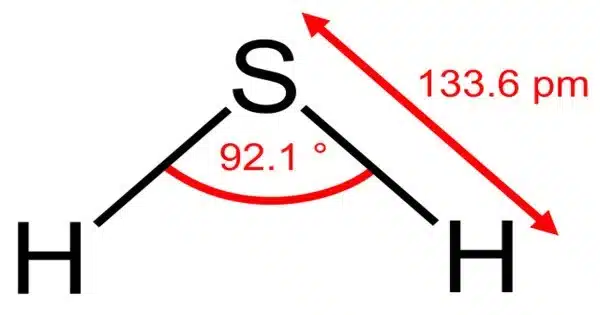
Hydrogen sulphide is a chemical substance having the formula H2S. It is a colourless chalcogen-hydride gas that is toxic, caustic, and combustible, with tiny levels in the ambient atmosphere emitting a characteristic rotten egg odour. stinkdamp is the underground mine gas word for foul-smelling hydrogen sulfide-rich gas mixes. It is made up of two hydrogen atoms and one sulphur atom. H2S is a naturally occurring gas that can be found in a variety of situations, including wetlands, volcanic areas, and some industrial operations.
Carl Wilhelm Scheele, a Swedish chemist, is credited with discovering the chemical composition of pure hydrogen sulphide in 1777. The Royal Society of Chemistry and the International Union of Pure and Applied Chemistry no longer support the spelling hydrogen sulphide for this chemical.
Properties
- Chemical formula: H2S
- Molar mass: 34.08 g·mol−1
- Appearance: Colorless gas
- Odor: Foul, pungent, like that of rotten eggs
- Density: 1.539 g.L−1 (0°C)
- Melting point: −85.5 °C (−121.9 °F; 187.7 K)
- Boiling point: −59.55 °C (−75.19 °F; 213.60 K)
- Solubility in water: 3.980 g dm−3 (at 20 °C) [4]
- Vapor pressure: 1740 kPa (at 21 °C)
- Acidity (pKa): 7.0
- Conjugate acid: Sulfonium
- Conjugate base: Bisulfide
Uses
While hydrogen sulphide can be dangerous, it also has some industrial applications. It is employed in the manufacturing of compounds such as sulfuric acid, as a reducing agent in organic chemistry, and in some laboratory applications.
Hydrogen sulphide is frequently created by the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is known as anaerobic digestion, and is carried out by sulfate-reducing microbes. It is also found in volcanic gases, natural gas deposits, and even in well-drawn water.
Toxicity
Hydrogen sulphide is hazardous to humans and most other animals because it inhibits cellular respiration in the same way that hydrogen cyanide does. When inhaled or consumed in large quantities, it causes fast organ destruction with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body generates modest amounts of this sulphide and its mineral salts and use it as a signalling molecule.