Mercury telluride (HgTe) is a chemical compound that contains both mercury and tellurium. It is a semimetal that belongs to the semiconductor material group II-VI. Mercury(II) telluride is another name for mercuric telluride.
HgTe occurs naturally as the mineral coloradoite. Bulk growth occurs in the presence of a high mercury vapour pressure from a mercury and tellurium melt. HgTe can also be grown epitaxially, using techniques such as sputtering or metalorganic vapour phase epitaxy.
Properties
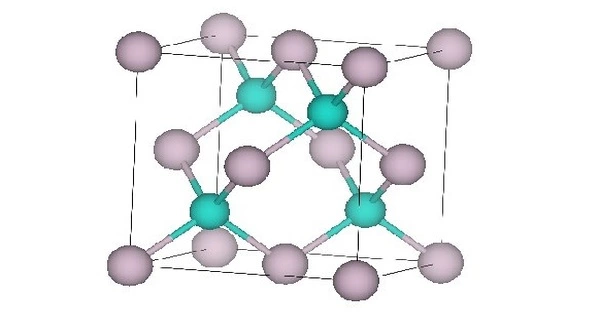
All properties are at standard temperature and pressure unless stated otherwise. The lattice parameter is about 0.646 nm in the cubic crystalline form. The bulk modulus is about 42.1 GPa. The thermal expansion coefficient is about 5.2×10-6/K. Static dielectric constant 20.8, dynamic dielectric constant 15.1. Thermal conductivity is low at 2.7 W·m2/(m·K). HgTe bonds are weak leading to low hardness values. Hardness 2.7×107 kg/m2.
- Chemical formula: HgTe
- Molar mass: 328.19 g/mol
- Appearance: near black cubic crystals
- Density: 8.1 g/cm3
- Melting point: 670°C
- Crystal structure: Sphalerite, cF8

Preparation
Mercury telluride was created through the direct reaction of mercury vapor and liquid tellurium. The compound was zone melted and annealed in mercury vapor at various mercury pressures. The anneal was discovered to change the composition near stoichiometry. The temperature was used to determine the Hall coefficient and resistivity.
Doping
Boron, aluminum, gallium, and indium can all be used to achieve N-type doping. Iron and iodine will also dope n-type. Because of the mercury vacancies, HgTe is naturally p-type. P-type doping can also be accomplished by incorporating zinc, copper, silver, or gold.
Topological insulation
In 2007, the first topological insulator was discovered: mercury telluride. Topological insulators cannot support an electric current in the bulk, but electronic states confined to the surface can act as charge carriers.
Chemistry
HgTe bonds are weak. Their enthalpy of formation, around 32kJ/mol, is less than one-third that of the related compound cadmium telluride. Acids such as hydrobromic acid can easily etch HgTe.
Uses
Mercury telluride and mercury telluride-cadmium telluride are semiconductors that have been studied as intrinsic infrared-detecting materials. Mercury telluride quantum dots provide complete coverage of low-loss fiber windows in colloidal nanocrystals for telecommunications.