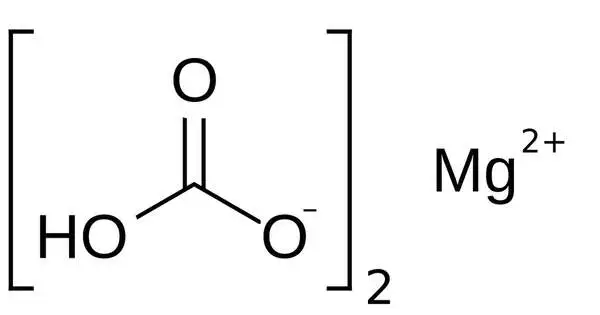
Magnesium bicarbonate, Mg(HCO3)2, is the bicarbonate salt of magnesium. It is an ionic molecule generated by the interaction of magnesium ions (Mg2+) and bicarbonate ions (HCO3). It can be produced by reacting dilute concentrations of carbonic acid (such as seltzer water) with magnesium hydroxide (milk of magnesia). This chemical is more usually encountered in solution form rather than in pure form as a solid.
Properties
- Solubility: It is highly soluble in water. When magnesium ions (Mg2+) and bicarbonate ions (HCO3-) are present in water, they can form soluble magnesium bicarbonate complexes.
- Stability: It is not stable in its solid form and tends to decompose into other compounds. This is why it is usually found in solution rather than as a solid salt.
- pH: When dissolved in water, magnesium bicarbonate can act as a weak base, contributing to the alkalinity of the solution. It can help to buffer or neutralize acidic conditions to some extent.
- Magnesium Source: It serves as a source of magnesium ions, which are essential for various biological processes in living organisms, including muscle and nerve function, bone health, and more.
Preparation
Magnesium bicarbonate is not readily available as a commercial product due to its instability. It is typically prepared by dissolving magnesium hydroxide (Mg(OH)2) or magnesium carbonate (MgCO3) in carbonated water (water containing dissolved carbon dioxide). The carbon dioxide reacts with the magnesium compound to form magnesium bicarbonate in solution. It can be prepared through the synthesis of magnesium acetate and sodium bicarbonate:
Mg(CH3COO)2 + 2 NaHCO3 → Mg(HCO3)2 + 2 CH3COONa
Magnesium bicarbonate exists only in aqueous solution. Magnesium does not form solid bicarbonate as does lithium. To produce it, a suspension of magnesium hydroxide is treated with pressurized carbon dioxide, producing a solution of magnesium bicarbonate:
Mg(OH)2 + 2 CO2 → Mg(HCO3)2
Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and water:
Mg2+ + 2 HCO3− → MgCO3 + CO2 + H2O
Magnesium bicarbonate can be formed when magnesium hydroxide (Mg(OH)2) or magnesium oxide (MgO) reacts with carbon dioxide (CO2) in water, leading to the formation of bicarbonate ions and the dissolution of magnesium ions. The resulting solution may have various concentrations of magnesium bicarbonate, depending on the reaction conditions and the solubility of magnesium compounds in water.
Application
Magnesium is an essential mineral for the human body, playing an important part in a variety of physiological activities such as muscle and neuron function, bone health, and blood pressure management. Magnesium bicarbonate may be used as a dietary supplement by certain persons to boost their magnesium intake.
Health Considerations
While magnesium is an essential mineral, consuming too much magnesium bicarbonate or magnesium supplements can cause magnesium toxicity, which can cause diarrhea, nausea, and other side effects. When utilizing magnesium bicarbonate as a dietary supplement, it is critical to adhere to the prescribed dosage guidelines.