Bismuth oxychloride is an inorganic bismuth compound with the formula BiOCl. It is a gleaming white solid that has been used since antiquity, most notably in ancient Egypt. Because of light wave interference caused by its plate-like structure, it has a pearly iridescent light reflectivity similar to nacre. It is also referred to as pearl white.
Bismuth oxychloride is a lustrous white powder. It is a white inorganic pigment that is commonly found in foundations, blushes, and other color cosmetics. Bismuth oxychloride is commonly available in two finishes: diamond and pearl. The diamond is more gleaming, while the pearl is more matte.
Properties
Bismuth oxychloride is an odorless white solid. It is a white crystalline basic salt approximately made usually by reaction of an acid solution of bismuth nitrate with a solution of sodium chloride and used chiefly as a pigment and a cosmetic.
- Chemical formula: BiOCl
- Density: 7.36 (meas.), 7.78 g/cm3 (calc.)
- Solubility in water: negligible
- Solubility: soluble in acids
- Crystal structure: Tetragonal, tP6

Structure
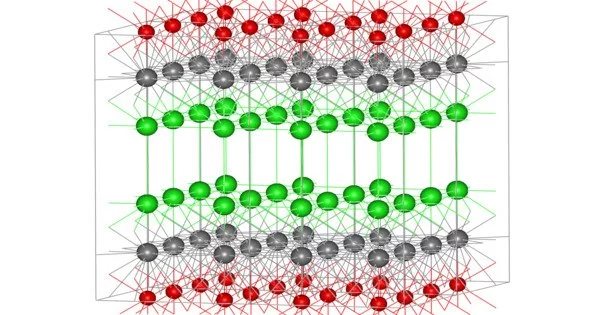
The structure of bismuth oxychloride can be thought of as consisting of layers of Cl−, Bi3+ and O2− ions. These ions are ordered as Cl-Bi-O-Bi-Cl-Cl-Bi-O-Bi-Cl, i.e., with alternating anions (Cl−, O2−) and cations (Bi3+).
Focusing on the coordination environment of the individual ions, the bismuth centers adopt a distorted square antiprismatic coordination geometry. The Bi3+ ion is coordinated to four chloride, forming one of the square faces, each at a distance of 3.06 Å from Bi, and four oxygen atoms forming the other square face, each at a distance of 2.32 Å from Bi. The oxygen atoms are tetrahedrally coordinated by four bismuth atoms.
Synthesis and reactions
BiOCl is formed during the reaction of bismuth chloride with water, i.e. the hydrolysis:
BiCl3 + H2O → BiOCl + 2 HCl
When heated above 600 °C, BiOCl converts to Bi24O31Cl10, called the “Arppe compound” which has a complex layer structure.
Preparation
Bismuth oxychloride is made by treating bismuth chloride with water and then drying the white precipitate so formed to expel a molecule of water:
BiCl3 + 2H2O → Bi(OH)2Cl + 2HCl
Bi(OH)2Cl → BiOCl + H2O
Also, the compound is prepared by treating a dilute nitric acid solution of bismuth nitrate with sodium chloride.
Use and occurrence
Bismuth occurs naturally in trace amounts. The majority of bismuth produced in the United States is a byproduct of the refining of lead, tin, copper, silver, and gold ores.
Since ancient Egypt, it has been used in cosmetics. It is a type of “pearly pigment” that can be found in eye shadow, hair sprays, powders, nail polishes, and other cosmetic products. Because of the plate-like structure of BiOCl, its suspensions have optical properties similar to nacre. C.I. 77163 is its cosmetic name.
BiOCl occurs naturally as the rare mineral bismoclite, which belongs to the matlockite mineral group. Bismuth oxynitrate, an analogous compound, is used as a white pigment.
Health Hazard
Dust contact causes mild eye irritation and can result in skin rashes. Bismuth is the least toxic of the elements in the periodic table, including lead, tin, antimony, and polonium. Bismuth is not safe for use in cosmetics on its own; it must be refined and combined with other elements to form bismuth oxychloride.