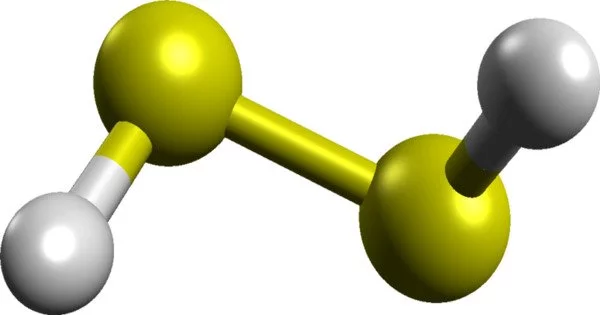
Hydrogen disulfide is an inorganic compound with the formula H2S2. This hydrogen chalcogenide is a pale yellow volatile liquid with a camphor-like odour. It easily decomposes into hydrogen sulphide (H2S) and elemental sulphur. It is a colourless gas with a distinct odour that has been described as resembling rotten eggs.
It is naturally produced by the decay of organic matter, such as in swamps, sewers, and volcanic gases. It can also be found in some natural gas and petroleum deposits. Because hydrogen sulphide is toxic, care should be taken when handling or working with it. In potentially hazardous environments, adequate ventilation, respiratory protection, and monitoring equipment should be used.
Properties
Hydrogen sulfide is a gas at room temperature and pressure. It is denser than air and can accumulate in low-lying, poorly ventilated areas. It is soluble in water, and its solubility increases with decreasing temperature. It can form acidic solutions in water, known as sulfhydric acid.
- Chemical formula: H2S2
- Molar mass: 66.14 g·mol−1
- Appearance: yellow liquid
- Density: 1.334 g cm−3
- Melting point: −89.6 °C (−129.3 °F; 183.6 K)
- Boiling point: 70.7 °C (159.3 °F; 343.8 K)
- Flash point: flammable
Structure
The structure of hydrogen disulfide is similar to that of hydrogen peroxide, with C2 point group symmetry. Both molecules are distinctly nonplanar. The dihedral angle is 90.6°, compared with 111.5° in H2O2. The H−S−S bond angle is 92°, close to 90° for unhybridized divalent sulfur.
Synthesis
Hydrogen disulfide can be synthesised by cracking polysulfanes (H2Sx) according to this idealized equation:
H2Sx → H2S2 + Sx−2
The main impurity is trisulfane (H2S3). The precursor polysulfane is produced by the reaction of hydrochloric acid with aqueous sodium polysulfide. The polysulfane precipitates as an oil.
Reactions
Upon contact with water or alcohols, hydrogen disulfide readily decomposes under ambient conditions to hydrogen sulfide and sulfur. It is more acidic than hydrogen sulfide, but the pKa has not been reported. In organosulfur chemistry, hydrogen disulfide adds to alkenes to give disulfides and thiols.
Application
Hydrogen sulfide has various industrial applications. It is used in the production of chemicals, including sulfuric acid, and as a reducing agent in various chemical reactions. It is also used in the mining industry for mineral processing.
Toxicity
Hydrogen sulfide is highly toxic and can be hazardous to human health even at low concentrations. It affects the central nervous system and can cause respiratory paralysis or death at high concentrations.