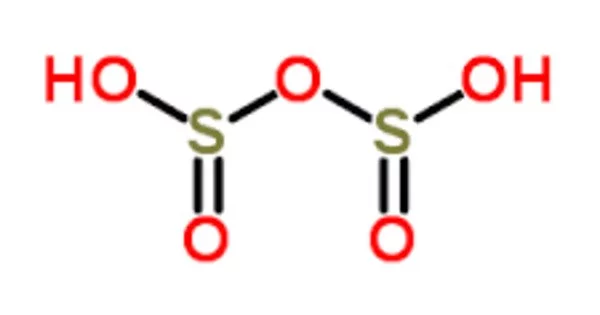
Disulfurous acid, also known as pyrosulfurous acid, is a sulfur oxoacid with the formula H2S2O5. It is an intermediate compound that is not well-studied or commonly encountered in its pure form. Disulfites and metabisulfites are the names given to the salts of disulfurous acid. Disulfurous acid, like sulfuric acid (H2SO3), is a phantom acid that does not exist in its free form. Disulfite, unlike disulfate (S2O7), has two sulfur atoms that are directly connected. The sulfur atom bonded to three oxygen atoms has an oxidation state of +5, while the other has an oxidation state of +3. However, it plays a role in certain chemical reactions and is relevant in the context of sulfur chemistry.
Properties
- Stability: It is relatively unstable and readily decomposes into sulfur dioxide (SO2) and water (H2O). The decomposition reaction can be triggered by heat, light, or exposure to strong acids.
- Acidic Nature: It is a weak acid and can release two protons (H+) in solution. It behaves as a reducing acid, meaning it can be oxidized to sulfuric acid or other sulfur compounds.
- Aqueous Solution: It can form a colorless solution in water. However, the solution is relatively unstable, and decomposition occurs over time, leading to the formation of sulfur dioxide gas and water.
Reactions
Disulfurous acid can react with various substances, including acids, bases, and oxidizing agents. For example, when disulfurous acid reacts with strong acids, it forms sulfur dioxide gas (SO2) and water. When it reacts with strong oxidizing agents, it can be oxidized to sulfuric acid (H2SO4).
Disulfurous acid is derived from sulfur dioxide (SO2) and water (H2O) and can be considered a derivative of sulfuric acid (H2SO4). When sulfur dioxide dissolves in water, it can react to form sulfurous acid (H2SO3), which is further oxidized to form disulfurous acid:
SO2 + H2O → H2SO3
2H2SO3 → H2S2O5
Disulfurous acid is an unstable compound and readily decomposes to release sulfur dioxide and water:
H2S2O5 → SO2 + H2O
The instability of disulfurous acid limits its isolation and study as a pure compound. Instead, it exists primarily as an intermediate species in certain chemical reactions, especially those involving sulfur compounds.
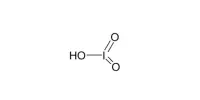
It’s worth noting that disulfurous acid should not be confused with sulfurous acid (H2SO3), which contains only one sulfur atom and is a well-known compound.
Reducing Agent
Disulfurous acid and its salts, known as dithionites or disulfites, are good reducing agents. They can undergo oxidation-reduction reactions and are often used in industrial processes for bleaching, reducing agents in chemical synthesis, and as antioxidants.