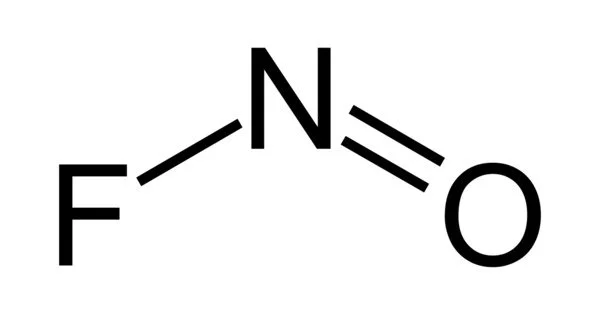
Nitrosyl fluoride (NOF) is a nitrosyl compound that is covalently bonded. The Nitrosyl fluoride molecule is made up of one or more Nitrogen atoms, one or more Oxygen atoms, and one or more Fluorine atoms, for a total of three atoms (s). Nitrosyl fluoride’s molecular weight is calculated by adding the atomic weights of each constituent element and multiplying by the number of atoms.
Properties
When pure, it is a colorless gas; impurities cause it to appear bluish; density 2.176 g/L; liquefies at -56°C; density of liquid 1.326g/mL at the boiling point; solidifies at -134°C; density of solid 1.719 g/cm3; reacts with water.
- Chemical formula: NOF
- Molar mass: 49.0045 g mol−1
- Appearance: Colourless gas
- Density: 2.657 mg mL−1(gas) 1.326 g/cm3(liquid)
- Melting point: −166 °C (−267 °F; 107 K)
- Boiling point: −72.4 °C (−98.3 °F; 200.8 K)
- Solubility in water: Reacts
Preparation
Nitrosyl fluoride may be prepared by the reaction of fluorine with nitric oxide:
F2 + 2NO → 2FNO
Nitrosyl fluoride also can be obtained by heating nitrosyl fluborate, NOBF4, and sodium fluoride:
NOBF4 + NaF → NaBF4 + FNO
Nitrosyl fluoborate required for the above preparation may be obtained by dissolving boric acid in 40% HF, concentrating the solution till it fumes, and purifying the NOBF4 formed by sublimation in a vacuum.
Nitrosyl fluoride also can be produced by the action of nitrosyl chloride with silver fluoride:
NOCl + AgF → FNO + AgCl
All preparations must be done in the complete absence of water.
Reactions
NOF is a highly reactive fluorinating agent that converts many metals to their fluorides, releasing nitric oxide in the process:
n NOF + M → MFn + n NO
NOF also fluorinates fluorides to form adducts that have a salt-like character, such as NOBF4.
Aqueous solutions of NOF are powerful solvents for metals, by a mechanism similar to that seen in aqua regia. Nitrosyl fluoride reacts with water to form nitrous acid, which then forms nitric acid:
NOF + H2O → HNO2 + HF
3 HNO2 → HNO3 + 2 NO + H2O
Nitrosyl fluoride can also convert alcohols to nitrites:
ROH + NOF → RONO + HF
It has a bent molecular shape: this can be rationalized in the VSEPR model in terms of the lone pair of electrons located on the N atom.
Uses
Nitrosyl fluoride is used as a solvent and as a fluorinating and nitrating agent in organic synthesis. It has also been proposed as an oxidizer in rocket propellants.