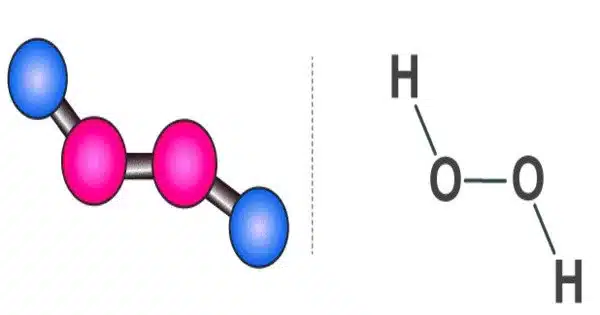
Hydrogen peroxide is a chemical compound with the formula H2O2. It is made up of two hydrogen atoms and two oxygen atoms. It is a very pale blue liquid that is slightly more viscous than water when pure. It is a pale blue liquid with a pungent odor that appears colorless in dilute solution. It is used as an oxidizer, bleaching agent, and antiseptic, usually in a dilute solution (3%-6% by weight) in water for consumer use, and in higher concentrations for industrial use. Because of its oxidizing and disinfecting properties, hydrogen peroxide is a powerful oxidizer that is commonly used for a variety of purposes. When heated, concentrated hydrogen peroxide, also known as “high-test peroxide,” decomposes explosively and has been used as a rocket propellant.
Properties
The boiling point of H2O2 has been extrapolated as being 150.2 °C (302.4 °F), approximately 50 °C (90 °F) higher than water. In practice, hydrogen peroxide will undergo potentially explosive thermal decomposition if heated to this temperature. It may be safely distilled at lower temperatures under reduced pressure.
- Molecular weight: 34.0147 g/mol
- Physical state: It is a liquid at standard conditions (25 degrees Celsius and 1 atmosphere pressure).
- Odor: It has a slightly pungent odor.
- Solubility: It is highly soluble in water. It forms a homogeneous solution with water in all proportions.
- Boiling point: It boils at around 150 degrees Celsius (302 degrees Fahrenheit).
- Density: The density of is approximately 1.45 g/cm³.
Reactivity
Hydrogen peroxide is a reactive oxygen species and the most basic peroxide, a compound with a single oxygen-oxygen bond. When exposed to light, it decomposes slowly into water and elemental oxygen, but quickly in the presence of organic or reactive compounds. It is typically stored in a dark bottle with a stabilizer in a weakly acidic solution to block light. The biological system, including the human body, contains hydrogen peroxide. Peroxidases are enzymes that use or decompose hydrogen peroxide.
Uses
Hydrogen peroxide has a wide range of applications, including:
- It can be used as an antiseptic to clean wounds and prevent infection.
- It is used as a bleaching agent for hair, textiles, and paper.
- It can be used to treat contaminated water by breaking down pollutants.
- It is used in high-concentration as a propellant in rocketry.
- It can be used as a cleaning agent for household surfaces.
- It is used in various chemical reactions and industrial processes.
Safety Precautions
Concentrated hydrogen peroxide can be hazardous and should be handled with care. It can cause skin burns and eye damage. Additionally, mixing hydrogen peroxide with certain substances, such as bleach or vinegar, can produce hazardous reactions, and should be avoided.