Any chemical compound with the empirical formula AlBrx is aluminium bromide. It appears as a bright yellow liquid. Because it is a colorless, sublimable hygroscopic solid, old samples are usually hydrated, primarily as aluminium tribromide hexahydrate (AlBr3•6H2O).
It appears in its anhydrous state as a white to yellowish-red compound with a lumpy solid and a pungent odor. It appears as a liquid in its watery form. It is extremely damaging to the eyes, mucous membranes, and skin. It is frequently utilized in the production of many compounds.
Properties
In its anhydrous state, it appears as a white to yellowish-red compound with a lumpy solid and a pungent odor. In its watery state, it appears as a liquid. Anhydrous density is 3.2 g/cm3 and hexahydrate density is 2.54 g/cm3. As an anhydrous, the melting point is 97.5 °C and as a hexahydrate, it is 93 °C.
- Molecular Weight: 266.69
- Appearance: White/pale pink solid
- Melting Point: 97.8 °C
- Boiling Point: 265 °C
- Density: 3.205 g/cm3
- Solubility in H2O: N/A

Structure
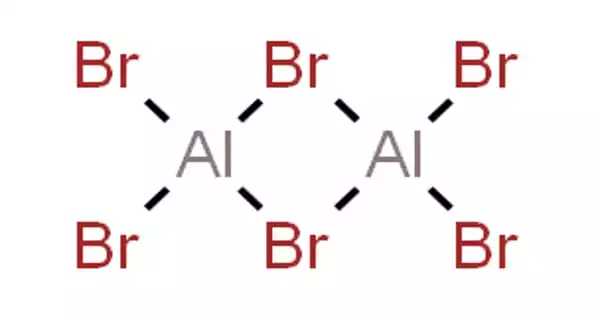
The dimeric form of aluminium tribromide (Al2Br6) predominates in the solid-state, in solutions in noncoordinating solvents (e.g. CS2), in the melt, and in the gas phase. Only at high temperatures do these dimers break up into monomers:
Al2Br6 → 2 AlBr3 ΔH°diss = 59 kJ/mol
The species aluminium monobromide forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature:
6/n “[AlBr]n” → Al2Br6 + 4 Al
This reaction is reversed at temperatures higher than 1000 °C. Theory suggests that the diatomic aluminium monobromide condenses to a dimer and then a tetrahedral cluster Al4Br4, akin to the analogous boron compound. Al2Br6 consists of two AlBr4 tetrahedra that share a common edge. The molecular symmetry is D2h.
The monomer AlBr3, observed only in the vapor, can be described as trigonal planar, D3h point group. The atomic hybridization of aluminium is often described as sp2. The Br-Al-Br bond angles are 120°.
Chemical Properties
At 100 °C, aluminium tribromide combines with carbon tetrachloride to produce carbon tetrabromide.
4 AlBr3 + 3 CCl4 → 4 AlCl3 + 3 CBr4
It reacts with phosgene gas to produce carbonyl bromide and aluminium chlorobromide.
AlBr3 + COCl2 → COBr2 + AlCl2Br
Water hydrolyzes Al2Br6 in line with the properties of Lewis acid. Furthermore, water hydrolysis occurs during the development of HBr and the production of the Al-OH-Br species. It also reacts fast with alcohol and carboxylic acids.
Reactions
A demonstration of the reaction of the exothermic reaction of the strong Lewis acid (Al2Br6) and strong Lewis base (H2O).
Aluminium tribromide reacts with carbon tetrachloride at 100 °C to form carbon tetrabromide:
4 AlBr3 + 3 CCl4 → 4 AlCl3 + 3 CBr4
and with phosgene yields carbonyl bromide and aluminium chlorobromide:
AlBr3 + COCl2 → COBr2 + AlCl2Br
Al2Cl6 is used as a catalyst for the Friedel-Crafts alkylation reaction. Related Lewis acid-promoted reactions include as epoxide ring openings and decomplexation of dienes from iron carbonyls. It is a stronger Lewis acid than the more common Al2Cl6.
Uses
- It is used as a catalyst in Friedel–Crafts reactions.
- It is used as a medication in the field of health.
- As it is a more powerful Lewis acid than Al2Cl6, it is employed in industries to create various substances.
- It acts as an electron pair acceptor to increase the reactivity of a substrate.
- It has a number of specific chemical uses in synthesis and extractive metallurgy.
Safety
Aluminium tribromide is a highly reactive material. Exposure through inhalation and swallowing can cause acute toxicity. Contact with skin and eyes can result in irritation, corrosion, and damage.