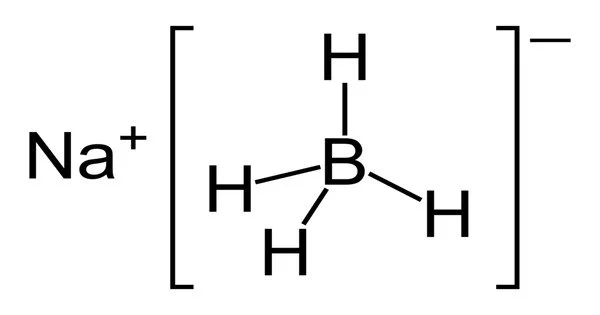
Sodium borohydride, abbreviated NaBH4, is an inorganic compound. This white solid, which is usually found as a powder, is a reducing agent used in chemistry, both in the laboratory and on a larger scale. It has been tested as a pretreatment for wood pulping, but it is too expensive to be commercialized. Although it slowly hydrolyzes, the compound is soluble in alcohols, certain ethers, and water.
H. I. Schlesinger, who led a team looking for volatile uranium compounds, discovered the compound in the 1940s. The findings of this wartime investigation were declassified and published in 1953.
Properties
Sodium borohydride is a white to gray-white odorless microcrystalline powder that frequently forms lumps. Recrystallization from warm (50 °C) diglyme can be used to purify it. Protic solvents such as water and lower alcohols dissolve sodium borohydride. It also reacts with these protic solvents to produce H2, but the reactions are relatively slow. At 20 °C, it takes nearly 90 minutes to completely decompose a methanol solution. It degrades in neutral or acidic aqueous solutions but remains stable at pH 14.
- Chemical formula: NaBH4
- Molar mass: 37.83 g/mol
- Appearance: white crystals hygroscopic
- Density: 1.07 g/cm3
- Melting point: 400 °C (752 °F; 673 K)(decomposes)
- Solubility in water: 550 g/L
- Solubility: soluble in liquid ammonia, amines, pyridine
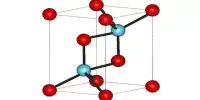
- Crystal structure: Cubic (NaCl), cF8

Synthesis and handling
For commercial NaBH4 production, the Brown-Schlesinger process and the Bayer process are the most popular methods. In the Brown-Schlesinger process sodium borohydride is industrially prepared from sodium hydride (produced by reacting Na and H2) and trimethyl borate at 250–270 °C:
B(OCH3)3 + 4 NaH → NaBH4 + 3 NaOCH3
Millions of kilograms are produced annually, far exceeding the production levels of any other hydride reducing agent. It can also be produced from inorganic borates, including borosilicate glass and borax (Na2B4O7):
Na2B4O7 + 16 Na + 8 H2 + 7 SiO2 → 4 NaBH4 + 7 Na2SiO3
Magnesium is a less expensive reductant, and could in principle be used instead:
8 MgH2 + Na2B4O7 + Na2CO3 → 4 NaBH4 + 8 MgO + CO2
and
2 MgH2 + NaBO2 → NaBH4 + 2 MgO
Applications
- Paper manufacture
Sodium borohydride is most commonly used in the production of sodium dithionite from sulfur dioxide: sodium dithionite is used as a bleaching agent for wood pulp and in the dyeing industry.
- Chemical synthesis
Sodium borohydride reacts with aldehydes and ketones to form the corresponding alcohols. This reaction is used to make antibiotics such as chloramphenicol, dihydrostreptomycin, and thiophenicol. In at least one step, sodium borohydride is used to prepare various steroids and vitamin A.
- Hydrogen storage
Sodium borohydride has been considered as a method of storing hydrogen for hydrogen-powered vehicles because it is safer and more efficient in terms of weight than most other alternatives. The hydrogen can be liberated by simply hydrolyzing the borohydride. However, this solution would require a low-cost and efficient method of recycling the hydrolysis product, sodium metaborate, back to the borohydride. As of 2007, no such procedure was available.
- Book conservation
Sodium borohydride can be used to reduce foxing in old books and documents. This treatment should only be performed by a skilled professional conservator/restorer because damage to paper can occur if the reducing agent is not applied correctly, such as over-bleaching and bubbling of paper.
Safety
Sodium borohydride is a flammable source of hydrogen or diborane. The solution of sodium borohydride in dimethylformamide can cause spontaneous ignition. Sodium borohydride bulk solutions are frequently prepared with corrosive sodium hydroxide.