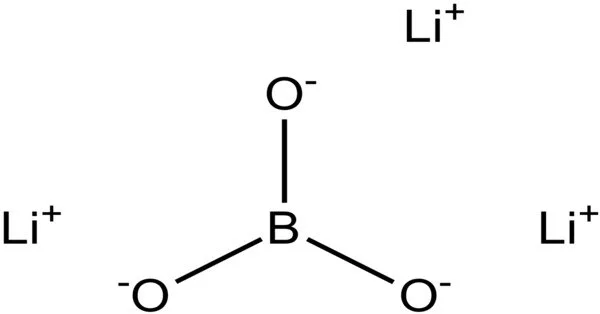
Lithium borate (Li2B4O7) is an inorganic compound that is commonly used in the field of optics. It is a colorless, transparent crystal with a high melting point and low thermal expansion coefficient, making it an excellent material for use in high-temperature applications.
Lithium borate has a wide range of uses in the field of optics, including as a component in the manufacture of glass and ceramic materials used in optical filters, lenses, and prisms. It is also used as a flux in the production of certain metals, as well as in the production of specialty glass products such as infrared windows and mirrors.
Properties:
- Physical Properties: It is a colorless, odorless crystalline solid that is soluble in water. It has a melting point of 925°C and a density of 2.47 g/cm3.
- Chemical Properties: It is a strong oxidizing agent and can react violently with reducing agents. It is stable under normal conditions but can decompose at high temperatures.
- Optical Properties: It has a high refractive index and low dispersion, making it useful as a component of optical glasses.
- Thermal Properties: It has a high thermal conductivity and a low coefficient of thermal expansion, making it useful in thermal shock-resistant applications.
- Electrical Properties: It is an insulator with a high dielectric constant and low dielectric loss, making it useful as a component of electrical ceramics and capacitors.
- Biological Properties: It has low toxicity and is not known to have any significant biological effects.
Applications
- Glass industry: It is used as a flux in the production of special glasses, such as borosilicate glass, which has high thermal resistance and low coefficient of thermal expansion. It is also used in the production of glass fibers and glass ceramics.
- Nuclear industry: It is used as a radiation shielding material and a neutron absorber in nuclear reactors.
- Analytical chemistry: It is used as a flux in the preparation of samples for X-ray fluorescence spectroscopy. It is also used in the analysis of minerals and rocks.
- Material science: It is used as a precursor for the synthesis of other borate compounds, such as lithium tetraborate, which has applications in the production of ceramics, semiconductors, and superconductors.
- Pharmaceutical industry: It has been investigated as a potential drug delivery system for cancer treatment. It has also been studied for its anti-inflammatory and anti-arthritic properties.
- Energy storage: It is being investigated as a potential electrolyte for lithium-ion batteries due to its high ionic conductivity and thermal stability.