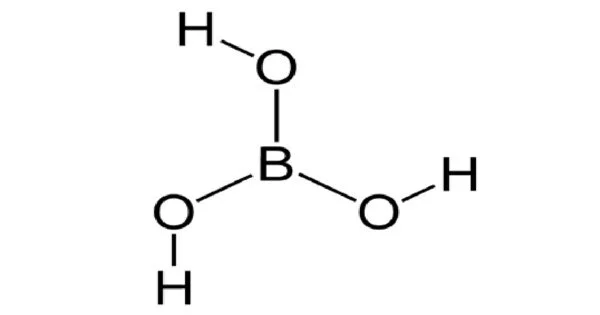
Boric acid, with the formula BO3H3 or B(OH)3, is a boron, oxygen, and hydrogen compound. Boracic acid is another name for hydrogen borate. It is commonly found in nature as the mineral sassolite in the form of colorless crystals or a white powder that dissolves in water. It is a weak acid that produces various borate anions and salts, as well as the ability to react with alcohols to form borate esters. It acts as an astringent. It is the dihydrogenborate conjugate acid.
Boric acid is a common antiseptic, insecticide, flame retardant, neutron absorber, and precursor to other boron compounds.
Properties
Boric acid is an odorless white solid. Its melting point 171°C. Sinks and mixes with water. It is soluble in water and does not have any characteristic odour. Under standard conditions, this compound exists either as a colourless crystal or in a white powdery form.
- Chemical formula: BH3O3
- Molar mass: 61.83 g·mol−1
- Appearance: White crystalline solid
- Density: 1.435 g/cm3
- Melting point: 170.9 °C (339.6 °F; 444.0 K)
- Boiling point: 300 °C (572 °F; 573 K)
- Solubility in water: 2.52 g/100 mL (0 °C); 27.53 g/100 mL (100 °C)
- Solubility in other solvents: Soluble in lower alcohols; very slightly soluble in acetone
The solubility of BO3H3 in water varies with temperature. Boric acid dissolves in water at a solubility of 57 g/L at 25 °C. When water is heated to 100 °C, the solubility of this compound increases to about 275 g/L. It is also worth noting that boric acid is only marginally soluble in pyridine and only slightly soluble in acetone. The borate anion is the conjugate base of boric acid.

History
Wilhelm Homberg (1652-1715) developed orthoboric acid from borax through the action of mineral acids and named it sal sedativum Hombergi (“sedative salt of Homberg”). However, boric acid and borates have been used for cleaning, preserving food, and other purposes since the time of the ancient Greeks.
Preparation
Boric acid may be prepared by reacting borax (sodium tetraborate decahydrate) with a mineral acid, such as hydrochloric acid:
Na2B4O7·10H2O + 2 HCl → 4 B(OH)3 + 2 NaCl + 5 H2O
It is also formed as a by product of hydrolysis of boron trihalides and diborane:
B2H6 + 6 H2O → 2 B(OH)3 + 6 H2
BX3 + 3 H2O → B(OH)3 + 3 HX (X = Cl, Br, I)
Application
As Medicine – Boric acid is widely used in medicine as an antiseptic for the treatment of minor cuts and burns. This compound is also found in medical dressings and salves. As an eyewash, very dilute solutions of boric acid can be used. Because of its antibacterial properties, boric acid can also be used to treat acne in humans. It can also be sprinkled into socks and shoes in powder form to prevent athlete’s foot (tinea pedis).
Some common uses are –
- It is used in the manufacture of textile fibreglass
- It is used to neutralize the active hydrofluoric acid
- It is used in electroplating
- It is used in the jewellery industry
- It is used as an antiseptic and antibacterial
- It is used on carrom boards as a dry lubricant.
Poisoning
This substance can poison you either acutely or chronically. Acute boric acid poisoning occurs when someone consumes powdered roach-killing items containing boric acid. A large dose of boric acid can have serious consequences in many areas of the body. The oesophagus and stomach are damaged for several weeks after swallowing boric acid. Complications may take months to manifest and may result in death.