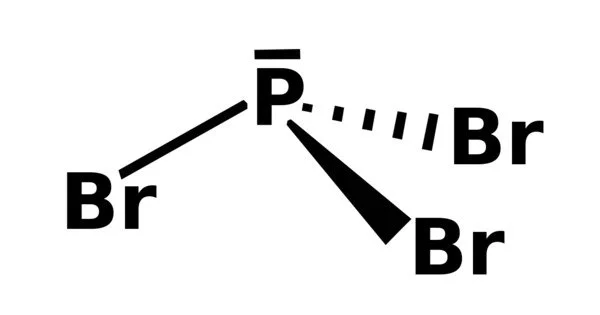
Phosphorus tribromide, also known as PBr3, is a colorless liquid with the formula PBr3. It has the appearance of a colorless fuming liquid with a strong odor. Because of hydrolysis, the liquid emits a pungent odor in moist air. It emits a pungent odor and fumes in the air as a result of hydrolysis. It is widely used in laboratories to convert alcohols to alkyl bromides. In the laboratory, it is used to convert alcohols to alkyl bromides.
Properties
It is a colorless liquid with sharp, penetrating order. It is used in chemical analysis, as a catalyst, and in the synthesis of other chemical compounds. It is used in the laboratory to convert alcohols to alkyl bromides.
- Chemical formula: PBr3
- Molar mass: 270.69 g/mol
- Appearance: clear, colorless liquid
- Density: 2.852 g/cm3
- Melting point: −41.5 °C (−42.7 °F; 231.7 K)
- Boiling point: 173.2 °C (343.8 °F; 446.3 K)
- Solubility in water: rapid hydrolysis
- Viscosity: 0.001302 Pas
- Molecular shape: trigonal pyramidal
Preparation
PBr3 is prepared by treating red phosphorus with bromine. An excess of phosphorus is used in order to prevent formation of PBr5:
2 P + 3 Br2 → 2 PBr3
Because the reaction is highly exothermic, it is often conducted in the presence of a diluent such as PBr.
Reactions
Phosphorus tribromide, like PCl3 and PF3, possesses both Lewis base and Lewis acid properties. For example, with a Lewis acid such as boron tribromide, it forms stable 1:1 adducts such as Br3B • PBr3. At the same time, PBr3 can act as an electrophile or Lewis acid in many of its reactions, such as those with amines.
The most important reaction of PBr3 is with alcohols, where it replaces an OH group with a bromine atom to produce alkyl bromide. All three bromides can be transferred.
PBr3 + 3 ROH → 3 RBr + HP(O)(OH)2
Applications
PBr3 is used as a reagent to convert alcohol to an alkyl halide. The main application of phosphorus tribromide is the conversion of primary or secondary alcohols to alkyl bromides, as described above. PBr3 typically yields higher than hydrobromic acid and avoids problems with carbocation rearrangement; for example, neopentyl bromide can be made from the alcohol in 60% yield.
Another application for PBr is as a catalyst for the -bromination of carboxylic acids. Although acyl bromides are less common than acyl chlorides, they are used as intermediates in Hell-Volhard-Zelinsky halogenation. Initially, PBr3 reacts with the carboxylic acid to form acyl bromide, which is more reactive to bromination.
Precautions
Phosphorus tribromide is a corrosive chemical that can irritate, burn, and damage the eyes. It is affected by breathing; it can irritate the nose, throat, and lungs, causing coughing, wheezing, and shortness of breath.
PBr3 produces corrosive HBr, which is toxic and reacts violently with water and alcohols.
PBr3 + 3 H2O → H3PO3 + 3 HBr
When working up by distillation in reactions that produce phosphorous acid as a by-product, be aware that this can decompose above about 160 °C to give phosphine, which can cause explosions when in contact with air.