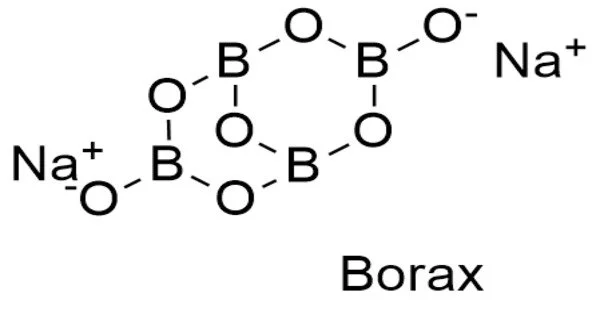
Borax is a salt (ionic compound), a hydrated sodium borate with the chemical formula Na2H20B4O17, also written Na2B4O7•10H2O. It is a powdery white substance that is also known as sodium borate, sodium tetraborate, or disodium tetraborate. It is a colorless crystalline solid that dissolves in water to form a basic solution. It is commonly available in powder or granular form and has numerous industrial and household applications, including pesticide, metal soldering flux, component of glass, enamel, and pottery glazes, tanning of skins and hides, artificial aging of wood, preservative against wood fungus, and pharmaceutic alkalizer.
It’s widely used as a household cleaner and a booster for laundry detergent. It’s a combination of boron, sodium, and oxygen. In chemical laboratories, it is used as a buffering agent.
Structure
From the chemical perspective, borax contains the [B4O5(OH)4]2− ion. In this structure, there are two four-coordinate boron centers and two three-coordinate boron centers.
Borax was first discovered in dry lake beds in Tibet. Native tincal from Tibet, Persia, and other parts of Asia was traded via the Silk Road to the Arabian Peninsula in the 8th century AD.

Physical properties
The crystalline decahydrate is a proton conductor at temperatures above 21 °C. Conductivity is maximum along the b axis.
- Chemical formula: B4O7Na2·10H2O or Na2B4O7·10H2O
- Molar mass: 381.37 (decahydrate)
- Appearance: white, crystalline solid
- Density: 1.73 g/cm3 (decahydrate, solid)
- Melting point: 743 °C (1,369 °F; 1,016 K) (anhydrous), 75 °C (decahydrate, decomposes)
- Boiling point: 1,575 °C (2,867 °F; 1,848 K) (anhydrous)
- Solubility in water: 31.7 g/L
Reactions
Borax is also easily converted to boric acid and other borates, which have many applications. Its reaction with hydrochloric acid to form boric acid is:
Na2B4O7·10H2O + 2 HCl → 4 H3BO3 + 2 NaCl + 5H2O
The “decahydrate” is sufficiently stable to find use as a primary standard for acid base titrimetry.
Molten borax dissolves many metal oxides to form glasses. This property is important for its uses in metallurgy and for the borax bead test of qualitative chemical analysis.
Occurrences
Natural sources of borax include evaporite deposits formed by the repeated evaporation of seasonal lakes. Turkey, Boron, California, and Searles Lake, California have the most commercially significant deposits. Furthermore, borax has been discovered in numerous other locations throughout the Southwest United States, the Atacama desert in Chile, newly discovered deposits in Bolivia, as well as in Tibet and Romania. Borax can also be synthesized from other boron compounds.
Uses
- Because it is toxic to ants, borax is used in pest control solutions. Worker ants will carry the borax to their nests and poison the rest of the colony because it is slow-acting.
- Borax can be found in a variety of household laundry and cleaning products, such as the 20 Mule Team Borax laundry booster, Boraxo powdered hand soap, and some tooth bleaching formulas.
- Borate ions are used in biochemical and chemical laboratories to make buffers, such as TBE buffer or the newer SB buffer or BBS buffer in coating procedures, for polyacrylamide gel electrophoresis of DNA and RNA.
- Borax has been used as a borate source to take advantage of borate’s ability to co-complex with other agents in water to form complex ions with various substances.
Health risks
If consumed alone, borax can cause nausea, vomiting, and diarrhea, and large amounts can result in shock and kidney failure. It is prohibited in food products in the United States. It can also irritate your skin and eyes, and if you breathe it in, it can harm your nose, throat, and lungs. If you are exposed to it frequently, it can cause rashes and may harm male reproductive organs.