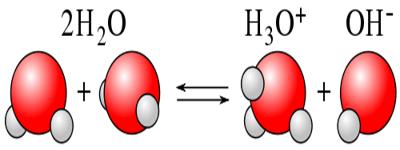
Ionic product of water: The dissociation of water, H2 O+ ↔ H+ + OH–
According to law of mass action, K = [H+] x [OH–] / [H2O]
or, K x [H2O] = [H+] x [OH–]
KW is called ionic product of water whose value is 1 x 10-4 at 25°C temperature. KW is essentially just an equilibrium constant for the reactions.