Dobereiner’s “triads” (1829) where groups of three similar elements were made such as Li, Na, K; Ca, Sr, Bo; or Cl, Br, I This was the forerunner of the idea of Groups.
Newlands “law of octaves” (1865) where around 60 known elements have been arranged in increasing R.A.M. Elements had similar properties to those 8 places before them and 8 places after them. This was the forerunner of the idea of Periods and led to the name Periodic Table.
Mendeleev’s table (1869)
Mendeleev arranged known elements in order of increasing R.A.M. but placed elements with similar properties underneath each other. Elements were arranged “periodically”. However, he left gaps where elements still to be discovered and predicted their properties.
Mendeleev’s predictions for Germanium
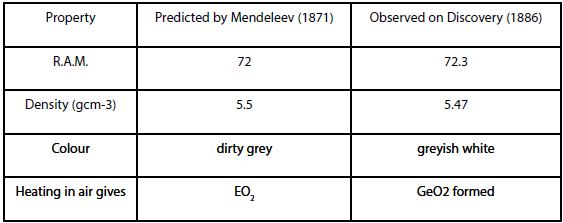
Predictions:
- “eka-aluminium”, discovered in 1875 and given the name Gallium
- “eka-boron”, discovered in 1879 and given the name Scandium
- “eka-silicon”, discovered in 1886 and given the name Germanium
Mendeleev also put elements in the “right place” e.g. Tellurium has greater R.A.M. than Iodine but Mendeleev realised I must come after Te and under Br.