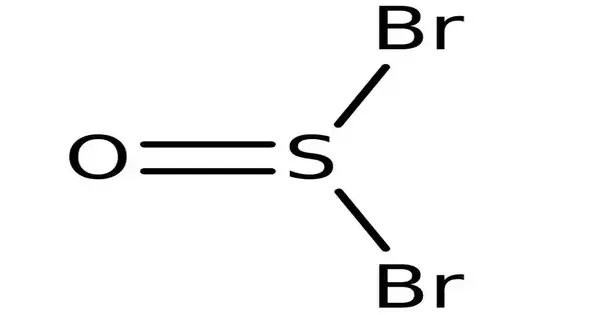
The chemical element SOBr2 is a thionyl bromide. Both of the halide atoms in this sulfinyl halide are chorines. In comparison to thionyl chloride, which is its chloride analog, it is less stable and less common. It consists of the molecules of a sulfinyl halide and chlorine.
Properties
Thionyl chloride is a fuming liquid that ranges in color from white to yellow and has a suffocatingly strong odor. At 79 °C, it reaches boiling point. The item is a lachrymator. The substance is poisonous and highly corrosive. Negative health consequences can result from breathing in high amounts for a short time or low concentrations for a long time.
- Chemical formula: SOBr2
- Molar mass: 207.87 g/mol
- Appearance: colorless liquid
- Density: 2.688 g/mL, liquid
- Melting point: −52 °C (−62 °F; 221 K)
- Boiling point: 68 °C (154 °F; 341 K) at 40 mmHg
- Solubility in water: decomposes
- Solubility: reacts in HBr, acetone, and alcohol, soluble in benzene, toluene, ether
- Molecular shape: trigonal pyramidal
Structure
The arrangement of atoms and the chemical bonds that hold them together make up a molecule’s chemical structure. Three bonds altogether are present in the thionyl bromide molecule (s) Three non-H bonds, one multiple bond, and one double bond are present (s).
Preparation
It is prepared by the action of hydrogen bromide on thionyl chloride, a characteristic reaction where a stronger acid is converted to a weaker acid:
SOCl2 + 2 HBr → SOBr2 + 2 HCl
Thionyl bromide will convert alcohols to alkyl bromides and can be used for brominations of certain α,β-unsaturated carbonyl compounds. It may occasionally be used as a solvent.
Uses
Thionyl Bromide is used in the preparation of carbon nanotube-phosphorylcholine polymer composite used in blood condition.
Safety
SOBr2 hydrolyzes readily in air to release dangerous fumes of sulfur dioxide and hydrogen bromide.
SOBr2 + H2O → SO2 + 2 HBr