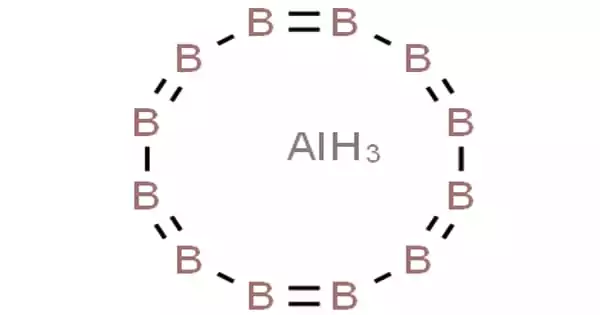
Aluminium dodecaboride (AlB12) is a super-hard chemical compound containing 17% aluminium by weight. It is a chemical compound composed of metal aluminum and nonmetal boron. At room temperature and pressure, it is a crimson solid that loses its surface shine when heated. It is stable in cold dilute acid but degrades in hot hydrochloric and nitric acid. It is created by combining fine aluminum and boron powders and reacting them with heat.
It is the most difficult boride in the aluminum-boron system, which also comprises AlB10, AlB4, AlB2, and AlB. It is one of two chemical compounds known as aluminum boride; the other is aluminum diboride. It’s a grinding compound that’s used to replace diamond or corundum.
Properties
Aluminium Dodecaboride is a chemical compound made from the metal aluminium and the non-metal boron. It is one of two chemical compounds that are commonly called aluminium boride. There are two crystalline forms, α-AlB12, and γ-AlB12. Both forms are very similar and consist of a framework with three-dimensional networks of B12 and B20 units. The phase β-AlB12 is now believed to be the ternary boride C2Al3B48.
- Molar mass: 156.714 g/mol
- Appearance: Yellow to black solid
- Density: 2.55 g/cm3
- Melting point: 2,070 °C (3,760 °F; 2,340 K)
- Solubility in water: insoluble
Preparation
The β-form can be prepared by the reaction of boron(III) oxide with sulfur and aluminum, then adding carbon to the mixture.
The majority of borides are crystals with a high melting point and hardness. Because of its chemical stability and wide variety of uses, it is frequently utilized in composite materials, semiconductors, and different fields of national defense, such as radiation protection. AlB12, for example, has a unique electrical structure and bonding properties. It is widely used in conductors and semiconductor materials because it can efficiently modify the conductivity of semiconductor materials. Aside from the aforesaid qualities, the boron concentration of AlB12 is extraordinarily high, reaching 82.8 percent, making it very promising as a neutron shielding material.
Traditional sintering processes are typically used to create ceramic powders. However, using this approach to synthesis ceramic powder takes a long time, consumes a lot of energy, and produces a lot of pollution. Self-propagating high-temperature synthesis (SHS) is a novel method for synthesis that involves the self-heating and self-conduction of high chemical reaction heat between reactants.
Uses
AlB12 extreme hardness makes it a favorable component of PCBN inserts, which are mainly used in cutting & grinding to replace diamond or corundum.