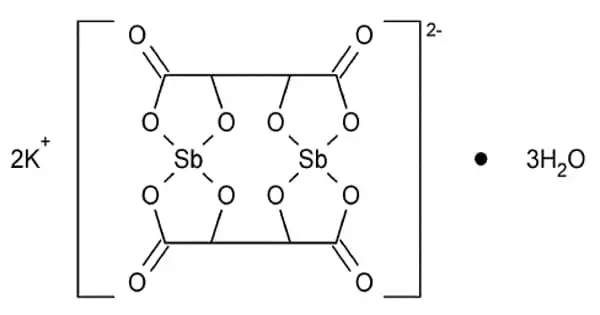
The formula for antimony potassium tartrate is K2Sb2(C4H2O6)2. The chemical has long been known as a strong emetic and has been used to treat schistosomiasis and leishmaniasis. It functions as a resolving agent. It is usually obtained as a hydrate.
Antimony potassium tartrate is a schistosomicide that may also be effective against other parasites. It possesses irritating emetic characteristics and, among other things, can cause deadly heart damage.
Properties
Antimony potassium tartrate is a colorless, odorless, poisonous crystalline salt that is used as a mordant to prepare fabrics and leather before dyeing and as a dyeing aid. Other applications as an insecticide.
- Chemical formula: K2Sb2(C4H2O6)2· 3 H2O
- Molar mass: 667.87 g/mol
- Appearance: white crystalline powder
- Density: 2.6 g/cm3
- Solubility in water: 8.3 g/100 mL (0 °C); 35.9 g/100 mL (100 °C)

Preparation, structure, reactions
Antimony potassium tartrate is prepared by treating a solution of potassium hydrogen tartrate and antimony trioxide:
2 KOH + Sb2O3 + (HOCHCO2H)2 → K2Sb2(C4H2O6)2 + 3 H2O
With an excess of tartaric acid, the monoanionic monoantimony salt is produced:
2 KOH + Sb2O3 + 4 (HOCHCO2H)2 → 2 KSb(C4H2O6)2 + 2 H2O
Several X-ray crystallographic studies have been conducted on antimony potassium tartrate. The core complex consists of an anionic dimer of antimony tartrate arranged in a big ring with the carbonyl groups pointing outwards. With two Sb(III) centers bound in deformed square pyramids, the complex possesses D2 molecular symmetry. The unit cell contains water and potassium ions, but they are not securely attached to the dimer. The anion is a common resolving agent.
Antimony potassium tartrate has been used as an emetic since the Middle Ages. Because the substance was deemed dangerous, a new method of administration was devised. Cups made from pure antimony were used to store wine for 24 hours and then the resulting solution of antimony potassium tartrate in wine was consumed in small portions until the wanted emetic effect was reached.
Medical Application
The first application of the compound to treat trypanosomiasis was tested in 1906, and the compound’s usage to treat other tropical diseases was investigated. Antimony potassium tartrate was first used to treat leishmania in 1913. The use of antimony potassium tartrate was phased out after the introduction of antimony(V) containing complexes such as sodium stibogluconate and meglumine antimoniate.
Antimonial medications became widely utilized after British physician John Brian Christopherson discovered in 1918 that antimony potassium tartrate might cure schistosomiasis. However, because the injection of antimony potassium tartrate caused severe adverse effects such as Adams–Stokes syndrome, alternate compounds were investigated. Antimony-based treatments became obsolete with the advent and subsequent widespread usage of praziquantel in the 1970s.