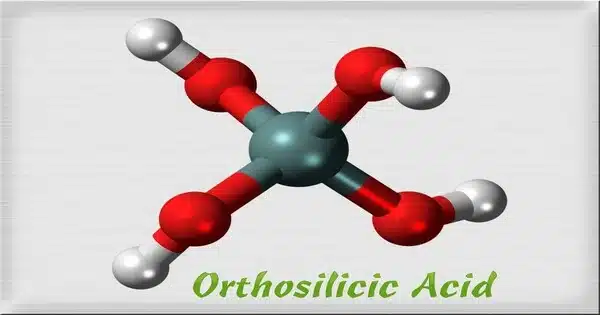
Orthosilicic acid is an inorganic chemical that has the formula Si(OH)4. It is a chemical compound composed of silicon, oxygen, and hydrogen. Although it is infrequently seen, it is a crucial molecule in silica and silicates, as well as the precursor to other silicic acids [H2xSiOx+2]n. It is a water-soluble silicon with a chemical formula generated from orthosilicate ions. Silicic acids have essential applications in biomineralization and technology. The substance is also known as soluble silica or monomeric silica.
It occurs naturally as a byproduct of silicate mineral weathering. It can also be found in natural liquids, such as mineral water.
Solubility
Orthosilicic acid is soluble in water, and its solubility increases with decreasing pH. It can be found in natural waters as a result of the weathering of rocks and minerals containing silicon.
Silicon Source
It is a bioavailable form of silicon, and it serves as a source of silicon for plants and some organisms. Plants take up orthosilicic acid through their roots, and it is believed to play a role in plant growth and development.
Isolation
Typically orthosilicic acid is assumed to be a product of the hydrolysis of its esters, Si(OR)4, where R stands for organyl group, as is practiced in sol-gel syntheses. These conditions are however too vigorous to allow isolation of the parent acid.
Orthosilicic acid can be produced by Pd-catalyzed hydrogenolysis of tetrabenzoxysilicon:
Si(OCH2Ph)4 + 4 H2 → Si(OH)4 + 4 PhCH3
The acid was crystallized from a solution of dimethylacetamide and tetrabutylammonium chloride. As established by X-ray crystallography, the chloride anions interact with the acid via hydrogen bonds. Otherwise, the structure consists of the expected tetrahedral silicon center.
Reactions
Silicic acid readily condenses to give “higher” silicic acids including disilicic (pyrosilicic) and cyclo-tetrasilicic acid, (−O−Si(OH)2−)4:
2 Si(OH)4 → O(Si(OH)3)2 + H2O
4 Si(OH)4 → (−O−Si(OH)2−)4 + 4 H2O
These derivatives have also been characterized crystallographically.
Applications
It is used in various industrial processes, such as in the production of silica gel, which has desiccant properties. Additionally, it finds applications in the electronics industry and as a component in some chemical formulations.