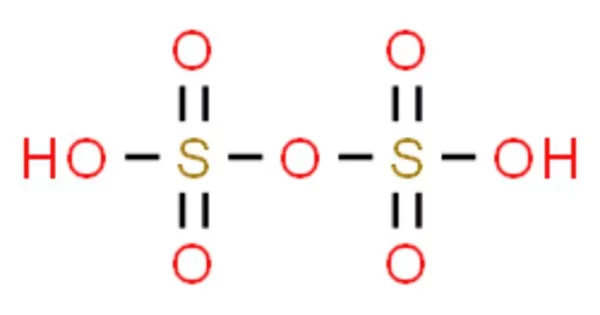
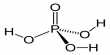
A sulfur oxoacid is disulfuric acid (alternative spelling disulphuric acid) or pyrosulfuric acid (alternative spelling pyrosulphuric acid), also known as oleum. It is a major constituent of fuming sulfuric acid, oleum, and is most commonly encountered by chemists. The molecule is made up of two SO2(OH) groups connected by an oxide, as confirmed by X-ray crystallography.
In the laboratory, sulfuric acid is commonly encountered as a fuming liquid with a viscous and oily consistency. It is a strong dehydrator that reacts violently with water, releasing heat and producing sulfuric acid. Because of this property, it can be used in a variety of industrial processes, including the production of detergents, dyes, and explosives.
Properties
- Acidic Properties: Disulfuric acid is a strong acid and can readily donate two protons (H+) when dissolved in water. It is more acidic than sulfuric acid.
- Fuming Nature: Disulfuric acid is highly hygroscopic, meaning it absorbs moisture from the air. It emits dense white fumes when exposed to moist air due to its reaction with water vapor.
- Oxidizing Agent: Disulfuric acid is a powerful oxidizing agent and can oxidize various organic and inorganic compounds. It can react violently with reducing agents and is used in some chemical reactions as an oxidizer.
- Corrosive Nature: Like other strong acids, disulfuric acid is corrosive to many materials, including metals, organic compounds, and even skin. It should be handled with extreme caution and appropriate safety measures.
- Strong Dehydrating Agent: Disulfuric acid is an excellent dehydrating agent. It can remove water molecules from various compounds and is often used in industrial processes to remove water from gases or to dehydrate organic compounds.
Disulfuric acid is the sulfuric acid equivalent of an acid anhydride. Each sulfuric acid unit’s mutual electron-withdrawing effects on its neighbor cause a significant increase in acidity. In the (anhydrous) sulfuric acid solvent system, disulfuric acid is strong enough to protonate “normal” sulfuric acid. There are disulfuric acid salts known as pyrosulfates, such as potassium pyrosulfate.
There are other related acids with the general formula H2O·(SO3)x though none can be isolated.
Disulfuric acid, due to its highly corrosive nature, can cause severe burns and damage to the skin, eyes, and respiratory system. It must be handled with care and should only be used in well-ventilated areas or under fume hoods. When working with this acid, safety equipment such as gloves, goggles, and lab coats should be worn.
Reactivity
Disulfuric acid is highly reactive and can react violently with many substances, including water, alcohols, amines, and some metals. It should be handled with care and stored properly to avoid accidents.
It is important to note that disulfuric acid is a hazardous substance, and its production, use, and disposal are governed by regulations and safety protocols designed to protect the environment and human health.