Photosensitized Reaction
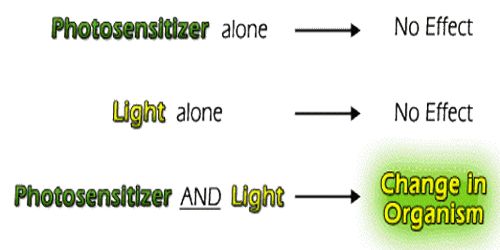
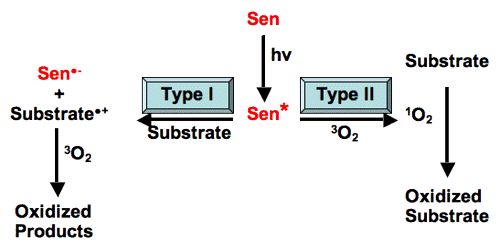
Photosensitization is a reaction to light that is mediated by a light-absorbing molecule, which is not the ultimate target. There are many substances that do not normally react when exposed to light. lf, however, another substance is added to it, photochemical reaction readily proceeds. The substance thus added does not undergo any chemical change. It merely absorbs light energy and then passes it on to the reactant substance. The added substance is called a photosensitizer since it sensitizes the reaction. The process is termed as photo-sensitization. The photosensitizer acts as a carrier of energy from the excited molecule to the reactant molecule. Photosensitization can involve reactions within living cells or tissues, or they can occur in pure chemical systems. In photobiology, we are concerned with the reactions in living systems. They are not usually consumed during photosensitization reactions. They return to their original state once the photosensitization reaction is complete.
If only hydrogen gas is irradiated by the ultraviolet light of λ = 253.70 nm the molecules do not dissociate to the atoms. But if the same radiation acts on hydrogen in presence of Hg-vapour, the hydrogen molecules undergo dissociation to the hydrogen atoms.
Hg + hv → Hg–
Hg+ + H2 → Hg + 2H
The radiation excites the mercury atoms and the excited mercury atoms transfer the energy to the hydrogen molecules by collision. The reaction occurs as a result of the transference of energy from photochemically excited molecules by collision- in this case from Hg to H2 molecules.
Another photosensitized reaction is the decomposition of oxalic acid in the presence of uranyl sulphate.
UO22+ + hv → UO22+*
UO22+* + (COOH)2 → UO22+ + CO2 + CO + H2O
The uranyl ion acts as a photosensitizer.
The decomposition of ozone in ordinary light by a trace of chlorine is an example of a photosensitized reaction. In ordinary photographic films or plates, silver bromide is the active ingredient which is decomposed by visible light of the shorter wavelength region. Thus red and orange bodies appear equally dark in the print. However, if suitable photosensitizes are mixed with the silver bromide the latter becomes sensitive to the entire part of the visible spectrum. can be photobleached, a chemical reaction following the absorption of light that produces a different molecular form, which does not absorb light, but this process typically competes with the photosensitization reaction. Such films are known as panchromatic films. These give a more natural and livelier picture than the orthochromatic films.
In a typical photosensitized reaction, as in the photodecomposition of ethylene to acetylene and hydrogen, a mixture of mercury vapor and ethylene is irradiated with a mercury lamp. The mercury atoms absorb the light energy, there being a suitable electronic transition in the atom that corresponds to the energy of the incident light. In colliding with ethylene molecules, the mercury atoms transfer the energy and are in turn deactivated to their initial energy state. The excited ethylene molecules subsequently undergo decomposition. Another mode of photosensitization observed in many reactions involves direct participation of the sensitizer in the reaction itself.