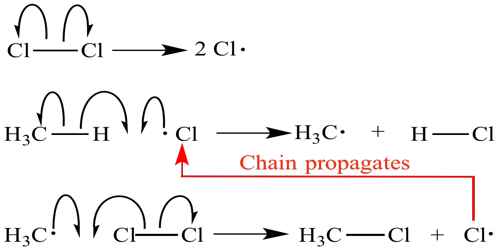
Chain Reactions
One type of complex reaction is the chain reaction. These reactions take place by way of a very reactive intermediate such as a free atom or a free radical – a group of atoms or an ion containing an unpaired electron. These reactive intermediates could be prepared by heating some molecules at a high temperature or by absorption of light of suitable wavelength. Once created they can react with other molecules to form a product plus yet another free atom or a free radical. This process, once initiated, can be repeated over and over again, making the reaction self-propagating. In chain reactions many molecules react for nets free atom or free radical formed. As a result the rate of the reaction rapidly increases unless the self-propagating steps are terminated by some mechanism. So, chain reaction is a chemical reaction or other process in which the products themselves promote or spread the reaction, which under certain conditions may accelerate dramatically.
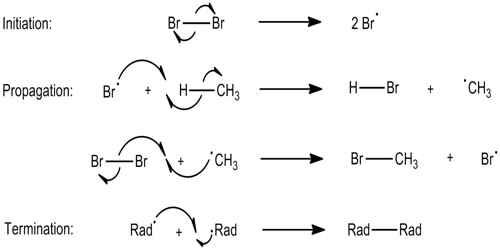
A chemical chain reaction proceeds by a sequence generally subdivided into three stages: (1) Initiation, is formed, usually through the action of an agent such as light, heat, or a catalyst. (2) Propagation, whereby the intermediate reacts with the original reactants, producing stable products and another intermediate, (3) Termination, which may be natural, as when all the reactants have been consumed or the containing vessel causes the chain carriers to recombine as fast as they are formed.