Rate Law and Mechanism of Reaction
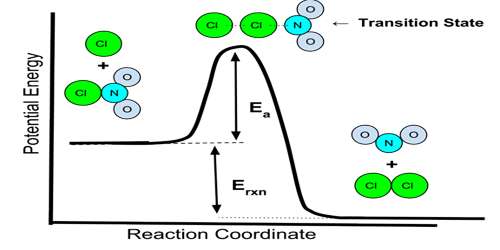
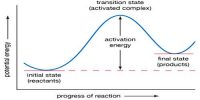
Mechanism of Reaction is the step by step sequence of elementary reactions by which overall chemical change occurs. It has been stated before that for a reaction to take place reacting molecules must collide with each other. Remembering that chemical reactions involve breaking and forming of bonds it is hard to imagine that these breaking and forming of bonds always take place instantaneously and in one step. It is believed that in most reactions more than one step is involved in the formation of the products from the reactants. Such speculations are arrived at by bringing together both theory and experiment.
Take for example the simple reaction:
NO2 (g) + CO (g) → NO (g) + CO2 (g) (overall reaction)
The reaction is experimentally found to be of second-order. This gas phase reaction is believed to take place in two steps:
NO2 (g) + NO2 (g) → NO3 (g) + NO (g) (elementary reaction)
NO3 (g) + CO (g) → NO2 (g) + CO2 (g) (elementary reaction)
Each step, called an elementary reaction, is a single molecular event. A sequence of such events leads to the overall or net reaction. The set of elementary reactions which lead to the overall or net reaction is called the mechanism of the reaction. The sum of the steps, or the elementary reaction equations, in the mechanism should yield the overall or net reaction equation.
According to the proposed mechanism for the above reaction two NO2 molecules collide to react and form one NO3 molecule and one NO molecule. NO3 is said to be a reaction intermediate. A reaction intermediate is a species produced during an elementary step of a reaction but does not appear in the net reaction equation. A reaction intermediate has a very short life and takes part in the subsequent step very fast. In most cases the reaction intermediate cannot be isolated and identified experimentally. In the above mechanism NO3 is the reaction intermediate, which reacts quickly with CO to produce NO2 and CO2. One can see that by aiding the above two elementary reaction equations the overall equation is obtained.
Let us consider the following example-
Carbon tetrachloride is obtained by the chlorination of chloroform, CHCl3.
Cl2 + CHCl3 → CCl4 I HCl
The following elementary steps have been proposed to constitute the mechanism of this gas phase reaction.
Cl2 ↔ 2Cl
Cl + CHCl3 → HCl + CCl3
Cl + CCl3 → CCl4
These equations add up to the overall equation.