Antimony trisulfide (Sb2S3) occurs in nature as the crystalline mineral stibnite and the amorphous red mineral (really a mineraloid) metastibnite. It is made for use in safety matches, military ammunition, explosives, and pyrotechnics. It is also utilized as a flame retardant in the manufacturing of ruby-colored glass and polymers. Historically, stibnite was employed as a grey pigment in 16th century paintings. It was also employed as the image sensitive photoconductor in vidicon camera tubes.
Antimony trisulfide is found in nature largely as the mineral stibnite, which is made up of two parallel Sb4S6 chains joined together. It is used in fireworks, some types of safety matches, as a pigment in paints, and in the production of ruby glass.
Properties
It is a black orthorhombic crystal or grayish-black powder; the compound is also available as an amorphous substance in a yellow-red variation; deformed octahedral configuration. It has a density of 4.64 g/cm3 for natural stibnite and 4.12 g/cm3 for the red modification; melts at 550°C; vaporizes at around 1150°C; is insoluble in water and acetic acid but soluble in hydrochloric acid and caustic soda solution; and is also soluble in alcohol, ammonium hydrosulfide, and potassium sulfide.
- Chemical formula: Sb2S3
- Molar mass: 339.715
- Appearance: grey / black orthorhombic crystal (stibnite)
- Density: 4.562g cm−3 (stibnite)
- Melting point: 550 °C (1,022 °F; 823 K) (stibnite)
- Boiling point: 1,150 °C (2,100 °F; 1,420 K)
- Solubility in water: 0.00017 g/100 mL (18 °C)

Preparation and reactions
Sb2S3 can be prepared from the elements at temperature 500–900 °C:
2 Sb + 3 S → Sb2S3
Sb2S3 is precipitated when H2S is passed through an acidified solution of Sb(III). This reaction has been used as a gravimetric method for determining antimony, bubbling H2S through a solution of Sb(III) compound in hot HCl deposits an orange form of Sb2S3 which turns black under the reaction conditions.
Sb2S3 is readily oxidised, reacting vigorously with oxidising agents. It burns in air with a blue flame. It reacts with incandescence with cadmium, magnesium and zinc chlorates. Mixtures of Sb2S3 and chlorates may explode.
In the extraction of antimony from antimony ores the alkaline sulfide process is employed where Sb2S3 reacts to form thioantimonate(III) salts (also called thioantimonite):
3 Na2S + Sb2S3 → 2 Na3SbS3
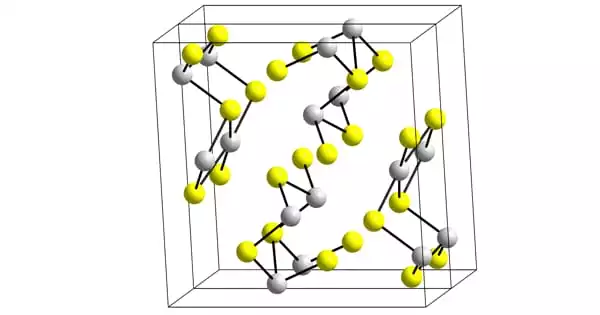
Structure
Stibnite, a black needle-like form of Sb2S3, has a structure composed of connected ribbons with antimony atoms in two distinct coordination environments, trigonal pyramidal and square pyramidal. Similar ribbons can be found in Bi2S3 and Sb2Se3. Metastibnite, the red form, is amorphous. Recent research reveals that there are several closely related temperature dependant stibnite structures, which have been labeled stibnite (I), the previously reported high temperature form, stibnite (II), and stibnite (III) (III). Another research demonstrates that the genuine antimony coordination polyhedra are SbS7, with (3+4) coordination at the M1 site and (5+2) coordination at the M2 site. These coordinations take into account the presence of secondary linkages. Some secondary links contribute cohesiveness and are linked with packing.
Applications
Antimony trisulfide occurs naturally as the crystalline mineral stibnite and the amorphous red mineral metastibnite. It is made for use in safety matches, military ammunition, explosives, and pyrotechnics. It is also utilized as a flame retardant in the manufacturing of ruby-colored glass and polymers. It is employed in the manufacture of pigments as well as as a rubber vulcanization agent.