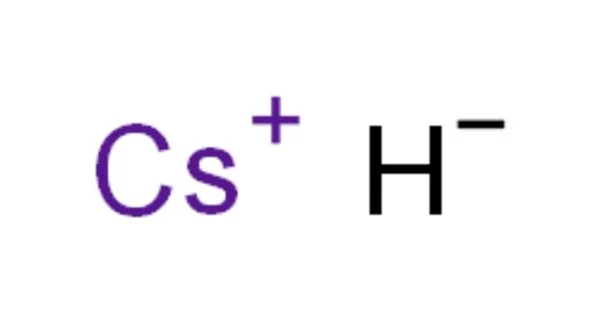
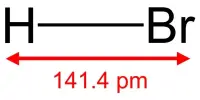
Caesium hydride, also known as cesium hydride (CsH), is a caesium and hydrogen compound. It is a hydride of an alkali metal. It was the first substance created by light-induced particle formation in metal vapor, and it showed promise in early studies of a caesium-based ion propulsion system. It is the most reactive and stable alkaline metal hydride. It is a strong superbase that reacts violently with water.
The three basic types of hydrides are saline (ionic), metallic, and covalent, depending on the type of chemical bond involved. Structure can also be used to identify a fourth type of hydride, dimeric (polymeric) hydride.
Properties
- Chemical formula: CsH
- Molar mass: 133.91339 g/mol
- Appearance: White or colorless crystals or powder
- Density: 3.42 g/cm3
- Melting point: ~170 °C (decomposes)
- Crystal structure: Face-centered cubic
- Coordination geometry: Octahedral.
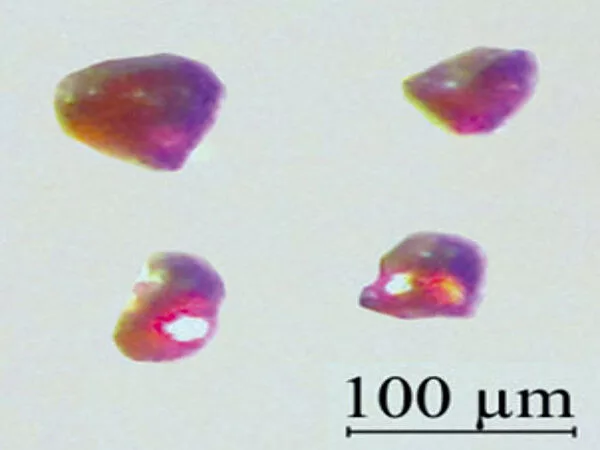
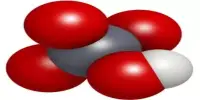
Crystal structure
CsH has the same structure as NaCl at room temperature and atmospheric pressure. Through interactions with an optically pumped caesium vapor, the caesium nuclei in CsH can be hyperpolarized, a process known as spin-exchange optical pumping (SEOP). SEOP can significantly boost the nuclear magnetic resonance (NMR) signal of caesium nuclei.
Synthesis
Caesium metal reacts directly with hydrogen gas to form the hydride caesium(I) hydride, CsH.
2Cs(s) + H2(g) → 2CsH(s)
It is extremely difficult to produce pure caesium hydride. Caesium hydride can be made by heating caesium carbonate and metallic magnesium in hydrogen at temperatures ranging from 580 to 620 degrees Celsius.