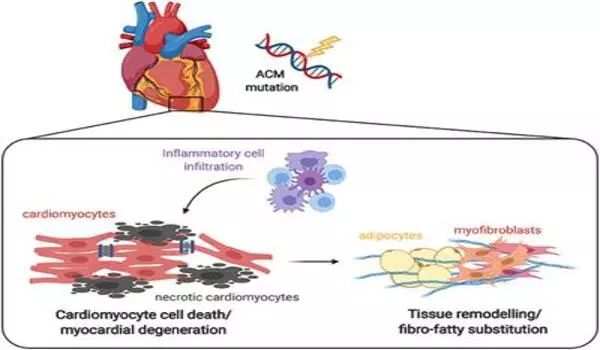
Arrhythmogenic cardiomyopathy (ACM) is a genetic heart disease characterized by abnormal heart rhythms and fatty and fibrous tissue replacing heart muscle. Desmoplakin is a protein that aids in the adhesion of heart muscle cells. ACM has been linked to mutations in the desmoplakin gene.
Researchers have identified a new mutation that leads to the cardiac disease arrhythmogenic cardiomyopathy (ACM). They assessed the effect of this mutation on heart muscle cells and obtained new insights into the underlying mechanism that causes the disease. The results of this study could contribute to the development of new treatments for ACM.
Researchers from the group of Eva van Rooij in collaboration with the UMC Utrecht identified a new mutation that leads to the cardiac disease arrhythmogenic cardiomyopathy (ACM). They assessed the effect of this mutation on heart muscle cells and obtained new insights into the underlying mechanism that causes the disease. The results of this study, published in Stem Cell Reports, could contribute to the development of new treatments for ACM.
We investigated the genetic material of an ACM patient and discovered a previously unknown mutation in the gene desmoplakin. We compared these heart muscle cells to the same heart muscle cells in which we repaired the mutation using CRISPR/Cas9.
van Kampen
Desmosomes
Millions of heart muscle cells contract to let the heart fulfill its pumping function. To make sure these contractions are executed well, it is important that the individual heart muscle cells communicate with each other. Therefore, heart muscle cells have to be tightly connected with each other. In a healthy heart, these connections are made up of complex protein structures that form a bridge between the cells, so called desmosomes.
When mutations occur in genes that contribute to these structures, this can lead to the development of arrhythmogenic cardiomyopathy (ACM). ACM is a progressive and often hereditary disease in which the heart fails to contract properly. Even though 1 in 5000 people develop ACM throughout life, a lot remains unknown about the disease and no effective treatment options exist to cure patients.
Discovery of a new mutation
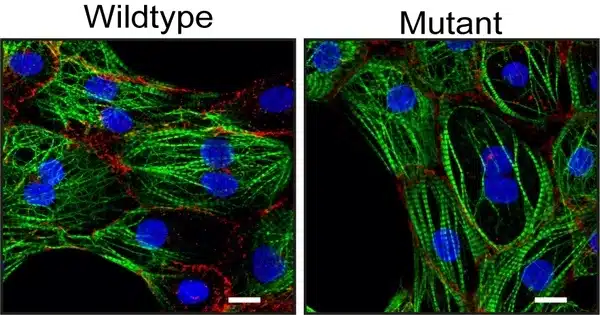
“We investigated the genetic material of an ACM patient and discovered a previously unknown mutation in the gene desmoplakin,” says Sebastiaan van Kampen, the study’s first author. Desmoplakin is one of the genes involved in the formation of desmosomes. The researchers cultured heart muscle cells from the patient in the lab to investigate the role of this mutation in the onset of ACM.
“We compared these heart muscle cells to the same heart muscle cells in which we repaired the mutation using CRISPR/Cas9,” says Van Kampen. The heart muscle cells with the mutation were found to be less tightly connected.”
In addition, the researchers discovered that these cells have fewer of ion channels. These channels are crucial for efficient propagation of the action potential, an electrical signal that stimulates contraction, between heart muscle cells. From this, they concluded that the new mutation can lead to ACM in patients.
Underlying mechanism
Multiple mutations are known to lead to ACM. Nevertheless, the underlying mechanism that leads to the disease remains largely unknown. To change this, the researchers used the cultured heart muscle cells from the patient as a model for the disease. They discovered that a protein named PITX2 is more highly expressed in the “diseased” heart muscle cells and that this protein is in part responsible for the loss of desmosomes and ion channels.
“When we removed the protein from the diseased heart muscle cells, the levels of ion channels and desmosomal proteins in the cells of the patient showed a remarkable recovery,” explains Van Kampen. The PITX2 protein thus plays an important role in the changes in the mutated heart muscle cells.