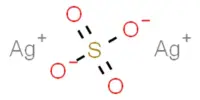
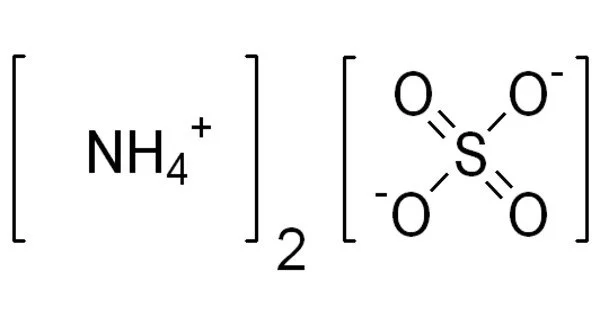Ammonium sulfate (NH4)2SO4 is an inorganic salt that has a variety of commercial applications. It is an inorganic sulfate salt formed by the reaction of sulfuric acid with two ammonia equivalents. Because of the high percentage of nitrogen in its composition, it is a salt that is commonly used as a soil fertilizer. It is composed of 21% nitrogen and 24% sulfur.
Ammonium sulfate is primarily used as a fertilizer for alkaline soils. The ammonium ion is released in the soil and forms a small amount of acid, lowering the pH balance while contributing essential nitrogen for plant growth. The main disadvantage of using ammonium sulfate is that it has a lower nitrogen content than ammonium nitrate, which raises transportation costs.
Properties
Ammonium sulfate becomes ferroelectric at temperatures below –49.5 °C. At room temperature it crystallises in the orthorhombic system. It t has no smell and dissolves in water easily. It does not dissolve in acetone. It appears as a crystalline solid white and has a salty taste.
- Molar mass: 132.14 g/mol
- Appearance: Fine white hygroscopic granules or crystals
- Density: 1.77 g/cm3
- Melting point: 235 to 280 °C (455 to 536 °F; 508 to 553 K) (decomposes)
- Solubility in water: 70.6 g per 100 g water (0 °C); 103.8 g per 100 g water (100 °C)
- Solubility: Insoluble in acetone, alcohol and ether
- Crystal structure: orthorhombic

Preparation
mmonium sulfate is found in nature as being a component of some minerals as mascagnite, which is found in volcanoes and coal fires.
Ammonium sulfate occurs naturally from the mineral mascagnite in volcanic fumaroles and due to coal fires on some dumps. Ammonium sulfate is made by treating ammonia with sulfuric acid:
2 NH3 + H2SO4 → (NH4)2SO4
At 60 °C, an ammonia gas and water vapor mixture is introduced into a reactor containing a saturated solution of ammonium sulfate and about 2 to 4 percent free sulfuric acid. To keep the solution acidic and maintain its level of free acid, concentrated sulfuric acid is added. The heat of reaction maintains the reactor temperature at 60°C. Spraying sulfuric acid into an ammonia-gas-filled reaction chamber can result in dry, powdered ammonium sulfate.
Gypsum (CaSO4•2H2O) is also used to make ammonium sulfate. An ammonium carbonate solution is enriched with finely divided gypsum. Calcium carbonate solidifies, leaving ammonium sulfate in the solution.
(NH4)2CO3 + CaSO4 → (NH4)2SO4 + CaCO3
Ammonium sulfate occurs naturally as the rare mineral mascagnite in volcanic fumaroles and due to coal fires on some dumps.
Reactions
Ammonium sulfate decomposes upon heating above 250 °C (482 °F), first forming ammonium bisulfate. Heating at higher temperatures results in decomposition into ammonia, nitrogen, sulfur dioxide, and water.
As a salt of a strong acid (H2SO4) and weak base (NH3), its solution is acidic; pH of 0.1 M solution is 5.5. In aqueous solution the reactions are those of NH4+ and SO4−2 ions. For example, addition of barium chloride, precipitates out barium sulfate. The filtrate on evaporation yields ammonium chloride.
When ammonium sulfate is mixed with equimolar solutions of metal sulfates and slowly evaporated, it forms many double salts (ammonium metal sulfates). Alums such as ferric ammonium sulfate are formed when trivalent metal ions are present. Ammonium cobaltous sulfate, ferrous diammonium sulfate, ammonium nickel sulfate (Tutton’s salts), and ammonium ceric sulfate are examples of double metal sulfates.
Airborne particles of evaporated ammonium sulfate account for roughly 30% of fine particulate pollution worldwide.
Uses
It is used as an agricultural spray adjuvant for water-soluble insecticides, herbicides, and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D (amine), glyphosate, and glufosinate herbicides.
- In the treatment of drinking water, ammonium sulfate is used in combination with chlorine to generate monochloramine for disinfection.
- Ammonium sulfate is used on a small scale in the preparation of other ammonium salts, especially ammonium persulfate.
- Ammonium sulfate has also been used in flame retardant compositions acting much like diammonium phosphate. As a flame retardant, it increases the combustion temperature of the material, decreases maximum weight loss rates, and causes an increase in the production of residue or char.
- Ammonium sulfate has been used as a wood preservative, but due to its hygroscopic nature, this use has been largely discontinued because of associated problems with metal fastener corrosion, dimensional instability, and finish failures.
Safety hazards
Ammonium sulfate is slightly toxic if inhaled and can cause eye irritation and respiratory irritation. It is toxic to aquatic life.