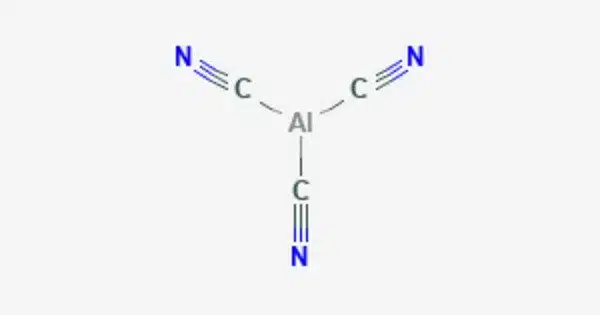
Aluminium cyanide is a metallic cyanide with a chemical formula of Al(CN)3. It is a coordination compound consisting of aluminum (Al) and cyanide ions (CN-). It is a white solid that undergoes hydrolysis to produce aluminium hydroxide and hydrogen cyanide. It is not commonly encountered and has limited practical applications, but it is of interest in the field of coordination chemistry.
Synthesis
Aluminium cyanide was first produced in 1924 as its ammoniate, Al(CN)3·5NH3, by reacting aluminium metal and mercury(II) cyanide in liquid ammonia to prevent hydrolysis.
2 Al + 3 Hg(CN)2 → 2 Al(CN)3 + 3 Hg
When the ammoniate contacts water, it produces aluminium hydroxide, ammonia, and ammonium cyanide.
The pure compound was produced in 2001 by the reaction of lithium tetrachloroaluminate and trimethylsilyl cyanide in diethyl ether and its crystals form an octahedral Prussian-blue-type structure.
Properties
- Molecular Weight: The molecular weight is approximately 105.98 g/mol.
- Physical State: It is likely to be a solid at room temperature.
- Stability: In general, cyanide compounds can be reactive, especially under certain conditions.
Toxicity
Cyanide compounds are generally highly toxic, and they can interfere with cellular respiration by inhibiting the activity of cytochrome c oxidase, an enzyme involved in the electron transport chain. Due to the potential toxicity of cyanide, handling and working with aluminum cyanide should be done with extreme caution, and it is not recommended for general laboratory use.
Cyanide ions interfere with cellular respiration by inhibiting the activity of enzymes that are essential for the process. Due to its toxicity, handling and use of aluminum cyanide should be carried out with extreme care, following strict safety protocols.