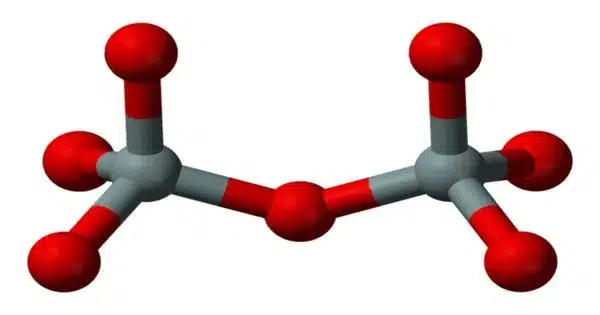
Sodium pyrosilicate is the chemical formula Na6Si2O7. It is a sodium silicate, specifically a pyrosilicate, which is a salt of the unstable pyrosilicic acid H6Si2O7. It’s also known as a water glass or a liquid glass. It is available in liquid and solid (powder or granules) forms.
It is soluble in water, and its solubility is determined by the ratio of sodium oxide (Na2O) to silicon dioxide (SiO2). The presence of hydroxide ions causes it to be alkaline in aqueous solutions. It is extensively employed as a binder in a variety of applications, including the fabrication of foundry moulds, cement mixtures, and as an adhesive.
Properties
- Chemical formula: Na6O7Si2
- Molar mass: 306.102 g·mol−1
- Appearance: It typically exists as white or colorless crystalline powder or granules.
- Solubility: It is soluble in water, producing an alkaline solution.
- pH: The aqueous solution is alkaline, with a pH of around 12-13.
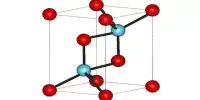
Structure
The anhydrous solid has the triclinic crystal structure, with space group P1 (a = 5.8007(8) Å, b = 11.5811(15) Å, c = 23.157(3) Å, α = 89.709(10)°, β = 88.915(11)°, γ = 89.004(11)°, V = 1555.1(4) Å3, Z = 8, Dx = 2.615 g·cm−3, μ(Mo‐Kα) = 7.94 cm−1). The Si2O6+7 anions are arranged in layers parallel to the (100) plane, with the sodium cations distributed in 24 distinct crystallographic positions, coordinated by 4 to 6 near oxygen atoms.
Some of the 4-coordinated sodium atoms can be interpreted as parallel columns of edge-sharing NaO4 tetrahedra. The columnar arrangement forms tunnels that house the remaining sodium cations. Twinning at a microscopic scale simulates a much larger monoclinic C centered lattice (V′ = 6220 Å3, Z = 32).
Applications
Because of its propensity to bind metal ions, soften water, and improve cleaning efficiency, sodium pyrosilicate is commonly used in laundry detergents and household cleansers. It is used in water treatment operations to avoid mineral deposits in pipes and equipment and to reduce scaling.
In the ceramic industry, sodium pyrosilicate is used as a binding agent and in glaze compositions to increase the stability and consistency of ceramic products. Because of its capacity to endure high temperatures, it has been employed in various fire-resistant materials and coatings.