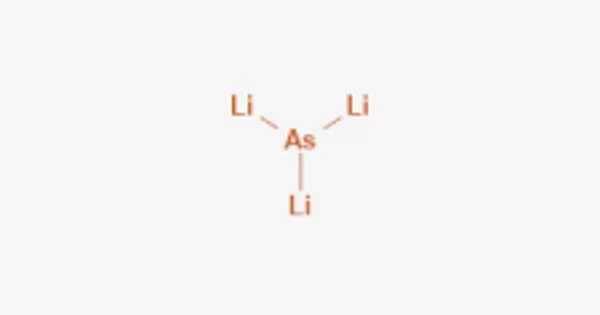
Lithium arsenide is a binary inorganic compound of lithium and arsenic with the chemical formula LiAs. It is an inorganic compound that consists of lithium (Li) and arsenic (As) atoms. It is a black solid with a crystalline structure and is a member of the lithium pnictides group.
It is a semiconductor material with a direct bandgap of about 1.8 eV at room temperature, making it a potential candidate for optoelectronic applications. It has also been studied as a potential anode material for lithium-ion batteries due to its high theoretical capacity.
Synthesis
Heating stoichiometric amounts of arsenic and lithium in an inert atmosphere:
Li + As → LiAs
Phsysical properties
Lithium arsenide forms monoclinic crystals, space group P21/c, cell parameters a = 0.579 nm, b = 0.524 nm, c = 1.070 nm, β = 117.4°, Z = 8.
- Chemical formula: LiAs
- Molar mass: 74.82 g/mol
- Appearance: LiAs is a grey or black solid that is insoluble in water.
- Crystal structure: LiAs has a zinc blende crystal structure, similar to that of diamond.
- Melting point: The melting point of LiAs is 1110 °C.
- Density: LiAs has a density of 3.83 g/cm³.
- Electrical conductivity: LiAs is a semiconductor and exhibits high electrical conductivity when doped with impurities.
- Magnetic properties: LiAs is not magnetic.
Applications
- Semiconductors: It is a potential candidate for use in semiconductors, as it has a high electron mobility and can conduct electricity efficiently. It could be used in electronic devices such as transistors and diodes.
- Solar cells: It has been explored as a potential material for use in solar cells, as it has a high absorption coefficient and could be used to create efficient and cost-effective solar panels.
- Optoelectronics: It could be used in optoelectronic devices such as light-emitting diodes (LEDs) and photodetectors, as it has good optical properties and could help improve the efficiency and performance of these devices.
- Thermoelectrics: It has also been studied for its potential use in thermoelectric materials, which can convert heat into electricity. It has good thermoelectric properties, and could be used to create more efficient thermoelectric devices.
Toxicity
Arsenic is a toxic substance, and LiAs should be handled with care to avoid exposure to arsenic.