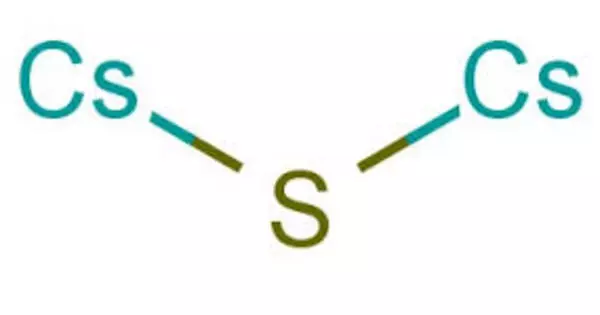
Caesium sulfide is an inorganic salt with the chemical formula Cs2S. It is a strong alkali in aqueous solution. Caesium sulfide emits rotten egg-smelling hydrogen sulfide into the air. Carl Plattner almost discovered caesium in 1846 while researching the mineral pollucite (caesium aluminium silicate). He could only account for 93% of the elements it contained before running out of material to analyze.
Caesium is a soft, gold-colored metal that is easily attacked by air and reacts explosively in water. The most common application for caesium compounds is as a drilling fluid. They are also used to make special optical glass, as a catalyst promoter, in vacuum tubes, and in radiation monitoring equipment.
Properties
- Chemical formula: Cs2S
- Molar mass: 297.876
- Appearance: white crystal
- Density: 4.19 g·cm−3
- Melting point: 480 °C
- Solubility in water: hydrolyses to form caesium bisulfide
- Solubility in ethanol and glycerol: soluble
- Crystal structure: cubic, anti-fluorite
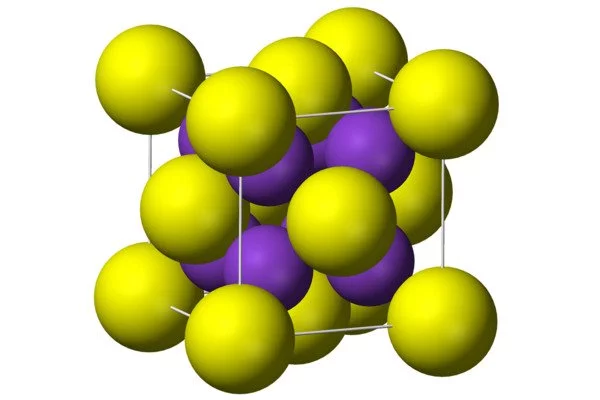
Production
Similar to sodium sulfide, anhydrous caesium sulfide can be produced by reacting caesium and sulfur in THF. It needs ammonia or naphthalene to react.
2Cs + S → Cs2S
By dissolving hydrogen sulfide into caesium hydroxide solution, it will produce caesium bisulfide, then it will produce caesium sulfide too.
CsOH + H2S → CsHS + H2O
CsHS + CsOH → Cs2S + H2O
Applications
It is used in industry as a catalyst promoter, increasing the capacity and hydrogenation of organic compounds of other metal oxides. Optical glasses are made using cesium nitrate. Cesium is occasionally used to remove oxygen traces from vacuum tubes and light bulbs. Cesium salts are used to reinforce different types of glass. Chloride is used in photovoltaic cells, optical instruments, and to boost the sensitivity of electron tubes. Cesium is a chemical element that is used in atomic clocks and, more recently, ion propulsion systems.
Health effects
Cesium can enter the body through breathing, drinking, or eating. Cesium levels in air are generally low, but radioactive cesium has been found in surface water and a variety of foods.
The amount of cesium in foods and beverages is determined by the release of radioactive cesium from nuclear power plants, primarily through accidents. These incidents have not occurred since the 1986 Chernobyl disaster. People who work in the nuclear power industry may be exposed to higher levels of cesium, but there are many precautionary measures that can be taken.