Aluminium acetylacetonate is a coordination compound containing aluminum, acetylacetonate (acac), and oxygen. It is typically used as a precursor for the synthesis of other aluminum compounds. It can be used in the production of ceramics and in catalysts for chemical reactions. It can also be used as a ligand in coordination chemistry.
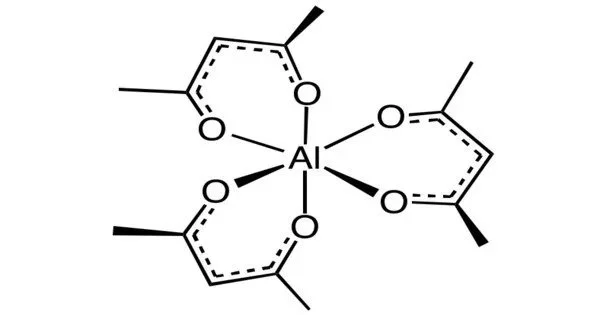
Aluminium acetylacetonate, also referred to as Al(acac)3, is a coordination complex with the formula Al(C5H7O2)3. It is an important organic intermediate (building block) to synthesize substituted acetylacetonate products. This aluminium complex with three acetylacetone ligands is used in research on Al-containing materials. The molecule has D3 symmetry, being isomorphous with other octahedral tris(acetylacetonate)s.
Properties
It is a white to yellow powder or crystals in appearance. It is stable and incompatible with strong oxidizing agents. It is a white powder that is soluble in polar solvents such as methanol and acetone. It is commonly used as a precursor in the synthesis of other aluminium compounds, as well as in the production of catalysts and as a reagent in organic synthesis.
- Chemical formula: Al(C5H7O2)3
- Molar mass: 324.31 g/mol
- Appearance: White solid
- Density: 1.42 g/cm3
- Melting point: 190-193 °C
- Boiling point: 315 °C
- Solubility in water: Low
Uses
Aluminum acetylacetonate is a coordination complex of aluminum and the ligand acetylacetonate (acac). It is often used as a precursor for the synthesis of other aluminum compounds and as a catalyst in organic chemistry. It is a white powder that is soluble in polar solvents such as methanol and ethyl acetate. It is also known as aluminum diacetylacetonate.
Aluminium acetylacetonate can be used as the precursor to crystalline aluminium oxide films using low-pressure metal-organic chemical vapour deposition. In horticulture it can also be used as a molluscicide.
Alumunium acetylacetonate may be used to prepare transparent superhydrophobic boehmite and silica films by sublimation, to deposit alumunium oxide films by chemical vapor deposition, to deposit alumunium oxide films by chemical vapor deposition, as a catalyst.