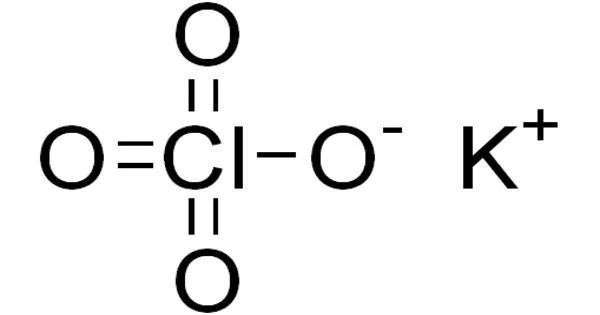
Potassium perchlorate is an inorganic salt with the chemical formula KClO4. It appears as a white crystalline solid. This salt, like other perchlorates, is a strong oxidizer, though it usually reacts slowly with organic substances. It is a strong oxidizer with the lowest solubility of the alkali metal perchlorates.
It has been used as a solid rocket propellant, though it has mostly been replaced in that application by the higher performance ammonium perchlorate. It works as a competitive inhibitor of iodine uptake by the thyroid gland, reducing thyroid hormone production. KClO4 has the lowest solubility of the alkali metal perchlorates (1.5 g in 100 mL of water at 25 °C).
Properties
KClO4 is an oxidizer in the sense that it exothermically transfers oxygen to combustible materials, greatly increasing their rate of combustion relative to that in air. This, usually obtained as a colorless, crystalline solid, is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers.
- Chemical formula: KClO4
- Molar mass: 138.55 g/mol
- Appearance: colourless/ white crystalline powder
- Density: 2.5239 g/cm3
- Melting point: 610 °C (1,130 °F; 883 K); decomposes from 400 °C
- Solubility in water: 0.76 g/100 mL (0 °C); 21.08 g/100 mL (100 °C)
- Solubility product (Ksp): 1.05·10−2
- Solubility: negligible in alcohol; insoluble in ether
- Solubility in ethanol: 47 mg/kg (0 °C); 120 mg/kg (25 °C)

Production
Industrially, potassium perchlorate is made by reacting an aqueous solution of sodium perchlorate with potassium chloride. This single precipitation reaction takes advantage of KClO4‘s low solubility, which is about 1/100 that of NaClO4 (209.6 g/100 mL at 25 °C).
It can also be produced by bubbling chlorine gas through a solution of potassium chlorate and potassium hydroxide, as well as by the reaction of perchloric acid with potassium hydroxide; however, due to the dangers of perchloric acid, this is not widely used.
Another method is to electrolyze a potassium chlorate solution, which causes KClO4 to form and precipitate at the anode. The low solubility of potassium chlorate and potassium perchlorate, the latter of which may precipitate onto the electrodes and obstruct the current, complicates this procedure.
Oxidizing properties
KClO4 is an oxidizer in the sense that it exothermically transfers oxygen to combustible materials, greatly increasing their rate of combustion relative to that in air. Thus, with glucose it gives carbon dioxide:
3 KClO4 + C6H12O6 → 6 H2O + 6 CO2 + 3 KCl
The explosive force of this and other such mixtures is based on the conversion of solid glucose into hot gaseous CO2. If the necessary confinement is provided, KClO4 produces a low explosive with sugar. Otherwise, such mixtures simply explode with an intense purple flame typical of potassium. The flash compositions used in firecrackers are typically a combination of aluminum powder and potassium perchlorate. This mixture, also known as flash powder, is used in both ground and air fireworks.
Potassium perchlorate can be used safely as an oxidizer in the presence of sulfur, whereas potassium chlorate cannot. Chlorate has a higher reactivity than perchlorates, which are kinetically poorer oxidants. Chlorate generates chloric acid, which is highly unstable and can cause the composition to ignite prematurely.
In commercial use, it is mixed 50/50 with potassium nitrate to make Pyrodex black powder substitute, and when not compressed within a muzzle loading firearm or cartridge, burns at a sufficiently slow rate to change its classification from “low explosive” to “flammable.”
Medicine use
Potassium perchlorate can be used as an antithyroid agent to treat hyperthyroidism, usually in combination with another medication. This application takes advantage of the similar ionic radius and hydrophilicity of perchlorate and iodide.