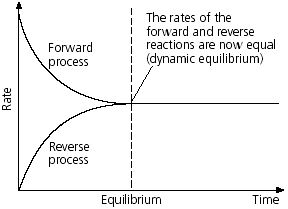
Dynamic equilibrium: When a reversible reaction reaches at equilibrium, then it seems to be stopped. Because, in this situation there seems to no change in concentration of reactant and products. But in actual the reactions never stop. In this state the rate of product formation in forward reaction is equal to the rate of reactor formation from product in backward reaction which is called dynamic equilibrium.