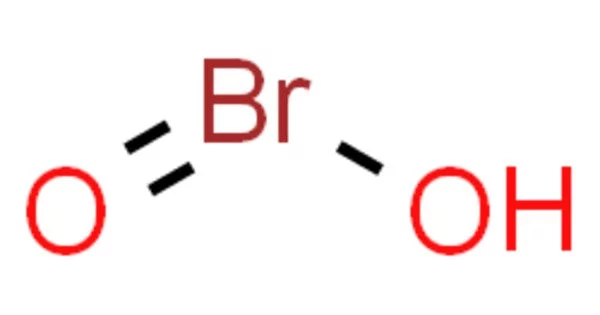
Bromous acid is an inorganic compound with the chemical formula HBrO2. Although salts of its conjugate base, bromites, have been isolated, it is an unstable compound. Bromites decompose to bromine in acidic solutions. It is not used directly in many applications due to its instability. However, it is involved in the synthesis of other bromine compounds and can serve as an intermediate in chemical reactions.
Properties
Bromous acid is a colorless or pale yellow solid at low temperatures. It is sparingly soluble in water, and its solutions are usually acidic.
- Chemical Formula: HBrO2
- Molecular Weight: 111.9 g/mol
- Acidic Nature: It is a weak acid. It can donate a proton (H+) to a base, forming the bromite ion (BrO2-). The acid dissociation constant (Ka) for bromous acid is relatively low.
- Oxidation State: It contains bromine in the +3 oxidation state, where bromine has a formal charge of +3.
Reactions
Bromous acid participates in various chemical reactions. It can undergo disproportionation, where it is simultaneously oxidized and reduced. For example:
3HBrO2 → HBrO3 + 2HBr
Structure
Bromous acid has a bent molecular geometry, similar to water (H2O). It consists of a central bromine atom (Br) bonded to two oxygen atoms (O) and a hydrogen atom (H).
H-O-Br=O
Stability
Bromous acid is generally unstable and tends to decompose into its constituent ions, particularly in the presence of heat or light. It is less stable compared to its counterparts, such as chloous acid (HClO2) and iodous acid (HIO2).
Bromous acid is a weak acid that can dissociate into hydrogen (H+) and bromite (BrO2-). It has one hydrogen atom, one bromine atom, and two oxygen atoms, according to its chemical formula. Bromous acid has a central bromine atom that is bonded to two oxygen atoms and one hydrogen atom.
However, bromous acid is unstable and readily decomposes to form bromine dioxide (BrO2) and water (H2O):
2HBrO2 → BrO2 + H2O
Application
Bromous acid is mostly used as an intermediate in chemical reactions and has few practical applications. It is primarily studied for its role in the formation of bromate (BrO3-) during the oxidation of bromide ions (Br-) in water treatment processes.