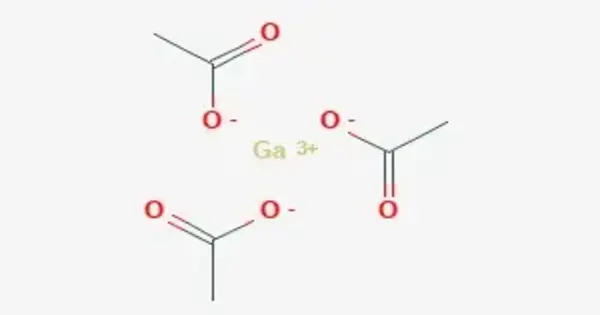
Gallium acetate is formed by the combination of gallium, a metal, and acetate, a salt or ester of acetic acid. It is a salt composed of a gallium atom trication and three acetate groups as anions where gallium exhibits the +3 oxidation state. It has a chemical formula of Ga(CH3COO)3 although it can be informally referred to as GaAc because Ac is an informal symbol for acetate. Gallium is moderately water-soluble and decomposes to gallium oxide when heated to around 70 °C.
Gallium acetate, like other acetate compounds, is a good precursor to ultra-pure compounds, catalysts and nanoscale materials. It is typically a white or colorless crystalline solid, and it is soluble in water. It is being considered as a substitute in de-icing compounds like calcium chloride and magnesium chloride. It has several applications, primarily in the field of chemistry and materials science. It is sometimes used as a precursor for the synthesis of other gallium compounds.
Properties
Gallium acetate is stable under normal conditions but may decompose when exposed to high temperatures. It is a source of gallium ions in various chemical reactions and synthesis processes. It is soluble in water and polar solvents due to the presence of acetate ions.
- Chemical formula: Ga(O2C2H3)3
- Molar mass: 246.85
- Appearance: white crystals
- Density: 1.57 g/cm/3
- Melting point: N/A
- Boiling point: 117.1C
Preparation
Gallium acetate can be formed using a neutralization reaction (acetic acid reacts with gallium oxide or gallium hydroxide):
6CH3COOH + Ga2O3 → 2Ga(CH3COO)3 + 3H2O
3CH3COOH + Ga(OH)3 → Ga(CH3COO)3 + 3H2O
Gallium can also be refluxed in acetic acid for several weeks to produce gallium acetate.
Applications
It can also be used in conjunction with acetylacetonate bis(thiosemicarbazone) to create radiogallium-acetylacetonate bis(thiosemicarbazone) complex. It can be used in tumor imaging. It is used in chemical research, particularly in gallium chemistry studies, and as a precursor for the synthesis of other gallium compounds. It also has potential applications in materials science and catalysis.
Toxicity
Like many gallium compounds, gallium acetate is considered to have low toxicity, but specific safety precautions should be taken when handling it, as with any chemical compound.