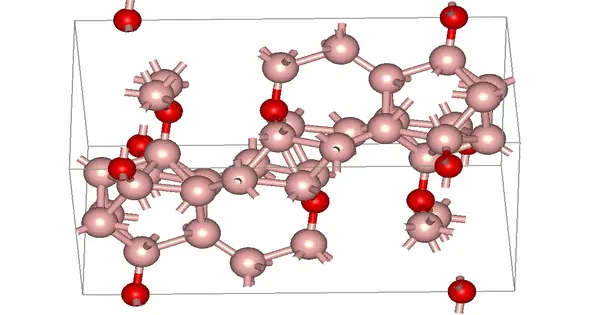
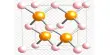
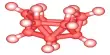
Boron suboxide (chemical formula B6O) is a solid compound with an eight-icosahedra structure at the apexes of the rhombohedral unit cell. Each icosahedron contains twelve boron atoms. There are two oxygen atoms in the interstices along the rhombohedral direction. B6O has a wide range of outstanding physical and chemical properties due to its short interatomic bond lengths and strongly covalent nature, including high hardness (similar to rhenium diboride and boron nitride), low mass density, high thermal conductivity, high chemical inertness, and excellent wear resistance.
Properties
B6O has a strong covalent nature and is easy to synthesize at temperatures above 1,973 K. Boron suboxide has also been reported to have a wide range of superior properties, including high hardness with low density, high mechanical strength, oxidation resistance up to high temperatures, and high chemical inertness.
- Chemical formula: B6O
- Molar mass: 80.865 g/mol
- Appearance: Reddish icosahedral twinned crystals
- Density: 2.56 g/cm3
- Melting point: 2,000 °C (3,630 °F; 2,270 K)
- Crystal structure: Rhombohedral, hR42
Preliminary first-principles ab initio density functional calculations of the structural properties of boron suboxide (B6O) suggest that the presence of a high electronegativity interstitial in the structure may improve bonding strength. The computational calculations confirm covalent bond shortening, which is thought to favor higher elastic constants and hardness values.
Synthesize
B6O can be made by either reducing B2O3 with boron or oxidizing boron with zinc oxide or other oxidants. These boron suboxide materials are generally oxygen deficient and non-stoichiometric (B6Ox, x<0.9), with poor crystallinity and very small grain size (less than 5 μm). High pressure used during B6O synthesis can significantly increase crystallinity, oxygen stoichiometry, and crystal size. In the reported methods for B6O synthesis, boron and B2O3 powder mixtures were typically used as starting materials.
Oxygen-deficient boron suboxide (B6Ox, x<0.9) might form icosahedral particles, which are neither single crystals nor quasicrystals, but twinned groups of twenty tetrahedral crystals.
B6O of the α-rhombohedral boron type has been investigated because of its ceramic nature (hardness, high melting point, chemical stability, and low density) as a new structural material. In addition to this, these borides have unique bonding not easily accessible by the usual valence theory. Although an X-ray emission spectroscopic method indicated a probable parameter range for the oxygen site of B6O, the correct oxygen position remained open to question until Rietveld analysis of X-ray diffraction profiles on B6O powders were first carried out successfully, even though these were preliminary investigations.
Applications
In recent years, there has been a lot of buzz about the potential applications of B6O as a wear-reduction coating for high-speed cutting tools, abrasives, and other high-wear applications. Commercial applications, however, have yet to be realized despite intensive research efforts.
This is due in part to the hot-pressed material’s low fracture toughness and the significant practical challenges associated with densifying stoichiometric B6O material with good crystallinity. Furthermore, many of the material’s mechanical properties were poorly understood until recently.