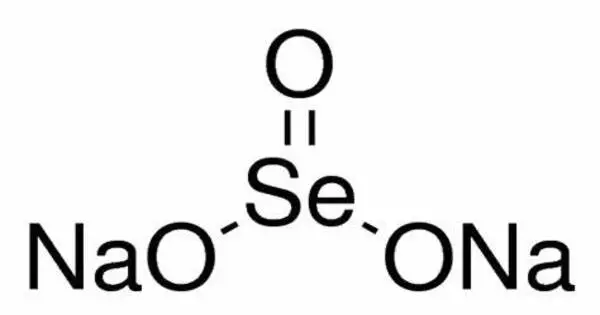
Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colorless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound. It is a form of selenium, an essential trace element for humans and animals.
Sodium selenite typically appears as a white or colorless crystalline solid. It is soluble in water, which makes it easy to incorporate into aqueous solutions. While selenium is an essential nutrient in small amounts, excessive intake can be toxic. Sodium selenite, when consumed in large quantities, can lead to selenium toxicity. It is important to use it with caution and adhere to recommended dosage guidelines.
Properties
- Chemical formula: Na2O3Se
- Molar mass: 172.948 g·mol−1
- Appearance: colourless solid
- Density: 3.1 g/cm3
- Melting point: decomposes at 710 °C
- Solubility in water: 85 g/100 mL (20 °C)
- Solubility: insoluble in ethanol
- Crystal structure: tetragonal
Applications
Together with the related barium and zinc selenites, sodium selenite is mainly used in the manufacture of colorless glass. The pink color imparted by these selenites cancels out the green color imparted by iron impurities.
- Nutritional Supplements: It is used as a dietary supplement to provide selenium, especially in regions where the soil is deficient in this essential element.
- Medicine: It has been investigated for its potential therapeutic applications, including its use in cancer treatment and prevention.
- Chemical Industry: It can be employed in the preparation of other selenium compounds and is used in some chemical processes.
Safety
Selenium is toxic in high concentrations. As sodium selenite, the chronic toxic dose for human beings was described as about 2.4 to 3 milligrams of selenium per day. Due to the potential toxicity of selenium, it is important to handle sodium selenite with care. Proper safety precautions should be taken to avoid ingestion, inhalation, or skin contact.