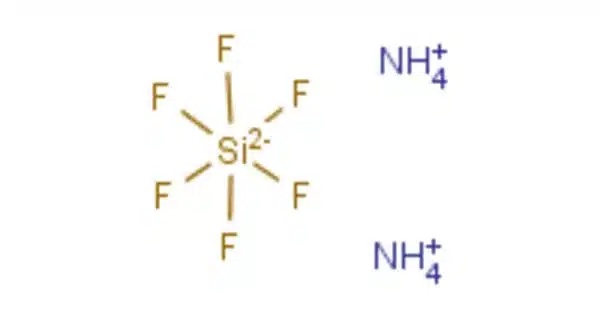
The formula for ammonium fluorosilicate is (NH4)2SiF6. It is a toxic chemical, as are all fluorosilicic acid salts. It is composed of white crystals with at least three polymorphs[6], and it can be found in nature as the rare minerals cryptohalite or bararite. It is a white crystalline solid that is extremely water soluble.
It is primarily used as a source of fluoride ions in a variety of industrial applications, including glass etching, metal surface treatment, and as a component in soldering and brazing fluxes. The compound is also used as a wood preservative, a disinfectant, and in some ceramics and glazes. In the electronics industry, it can be used in the manufacturing of semiconductors and as an additive in the production of optical fibers.
Properties
- Chemical formula: (NH4)2[SiF6]
- Appearance: White crystals
- Density: 2.0 g cm−3
- Melting point: 100 °C (212 °F; 373 K) (decomposes)
- Solubility: dissolves in water and alcohol
Chemical properties and health hazards
Although ammonium fluorosilicate is noncombustible, it will emit hazardous fumes in the event of a fire, including hydrogen fluoride, silicon tetrafluoride, and nitrogen oxides. It corrodes aluminum. Ammonium fluorosilicate dissolves in water to form an acid solution.
Inhaling dust can cause pulmonary irritation and even death. Ingestion can also be deadly. Contact with dust causes irritation of the eyes, as well as irritation or ulceration of the skin.
Natural occurrence
This chemical makes rare appearances in nature. It is found as a sublimation product of fumaroles and coal fires. As a mineral, it is either called cryptohalite or bararite, the two being two polymorphs of the compound.
Uses
Ammonium fluorosilicate finds use as a disinfectant, and it is useful in etching glass, metal casting, and electroplating. It is also used to help neutralize washing machine water as laundry sour.
- Glass etching: It is used in the manufacturing of glass as an etching agent.
- Wood preservation: It can be used as a wood preservative to protect against fungal decay and insect infestation.
- Aluminum refining: It is sometimes used in the aluminum industry as a flux or refining agent.
- Catalyst: It can act as a catalyst in certain chemical reactions.
- Electroplating: It can be used in electroplating processes to improve adhesion and corrosion resistance.
Safety Considerations
Due to its toxicity, ammonium fluorosilicate should be handled with caution. This compound can irritate the skin, eyes, and respiratory system. Inhaling its dust or fumes should be avoided, and proper ventilation and personal protective equipment (such as gloves and goggles) should be used when working with it.